New insights into human female reproductive tract development
- PMID: 28918284
- PMCID: PMC5712241
- DOI: 10.1016/j.diff.2017.08.002
New insights into human female reproductive tract development
Abstract
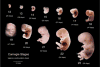
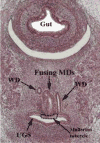
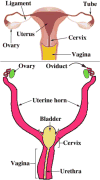
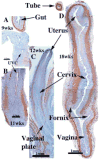
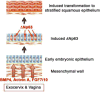
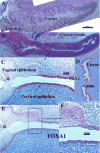
We present a detailed review of the embryonic and fetal development of the human female reproductive tract utilizing specimens from the 5th through the 22nd gestational week. Hematoxylin and eosin (H&E) as well as immunohistochemical stains were used to study the development of the human uterine tube, endometrium, myometrium, uterine cervix and vagina. Our study revisits and updates the classical reports of Koff (1933) and Bulmer (1957) and presents new data on development of human vaginal epithelium. Koff proposed that the upper 4/5ths of the vagina is derived from Müllerian epithelium and the lower 1/5th derived from urogenital sinus epithelium, while Bulmer proposed that vaginal epithelium derives solely from urogenital sinus epithelium. These conclusions were based entirely upon H&E stained sections. A central player in human vaginal epithelial development is the solid vaginal plate, which arises from the uterovaginal canal (fused Müllerian ducts) cranially and squamous epithelium of urogenital sinus caudally. Since Müllerian and urogenital sinus epithelium cannot be unequivocally identified in H&E stained sections, we used immunostaining for PAX2 (reactive with Müllerian epithelium) and FOXA1 (reactive with urogenital sinus epithelium). By this technique, the PAX2/FOXA1 boundary was located at the extreme caudal aspect of the vaginal plate at 12 weeks. During the ensuing weeks, the PAX2/FOXA1 boundary progressively extended cranially such that by 21 weeks the entire vaginal epithelium was FOXA1-reactive and PAX2-negative. This observation supports Bulmer's proposal that human vaginal epithelium derives solely from urogenital sinus epithelium. Clearly, the development of the human vagina is far more complex than previously envisioned and appears to be distinctly different in many respects from mouse vaginal development.
Keywords: Cervix; Human Müllerian duct; Urogenital sinus; Uterovaginal canal; Uterus; Vagina.
Copyright © 2017 International Society of Differentiation. Published by Elsevier B.V. All rights reserved.
Figures
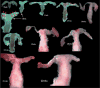













Similar articles
-
Development of the human female reproductive tract.Differentiation. 2018 Sep-Oct;103:46-65. doi: 10.1016/j.diff.2018.09.001. Epub 2018 Sep 6. Differentiation. 2018. PMID: 30236463 Free PMC article. Review.
-
Molecular mechanisms of development of the human fetal female reproductive tract.Differentiation. 2017 Sep-Oct;97:54-72. doi: 10.1016/j.diff.2017.07.003. Epub 2017 Jul 29. Differentiation. 2017. PMID: 29053991 Free PMC article.
-
Mouse vaginal development with lateral enlargement at late embryonic stages and caudal elongation after birth.Congenit Anom (Kyoto). 2023 Mar;63(2):30-39. doi: 10.1111/cga.12502. Epub 2022 Dec 25. Congenit Anom (Kyoto). 2023. PMID: 36517931
-
Immunohistochemical expression analysis of the human fetal lower urogenital tract.Differentiation. 2018 Sep-Oct;103:100-119. doi: 10.1016/j.diff.2018.09.004. Epub 2018 Sep 19. Differentiation. 2018. PMID: 30287094 Free PMC article.
-
Revisiting old vaginal topics: conversion of the Müllerian vagina and origin of the "sinus" vagina.Int J Dev Biol. 2009;53(7):925-34. doi: 10.1387/ijdb.082846yc. Int J Dev Biol. 2009. PMID: 19598112 Review.
Cited by
-
Altered Expression of Candidate Genes in Mayer-Rokitansky-Küster-Hauser Syndrome May Influence Vaginal Keratinocytes Biology: A Focus on Protein Kinase X.Biology (Basel). 2021 May 21;10(6):450. doi: 10.3390/biology10060450. Biology (Basel). 2021. PMID: 34063745 Free PMC article.
-
Protein-protein interaction network analysis applied to DNA copy number profiling suggests new perspectives on the aetiology of Mayer-Rokitansky-Küster-Hauser syndrome.Sci Rep. 2021 Jan 11;11(1):448. doi: 10.1038/s41598-020-79827-5. Sci Rep. 2021. PMID: 33432050 Free PMC article.
-
Decoding Müllerian Duct Epithelial Regionalization.Mol Reprod Dev. 2025 Feb;92(2):e70018. doi: 10.1002/mrd.70018. Mol Reprod Dev. 2025. PMID: 39994938 Free PMC article. Review.
-
Animal Models and Alternatives in Vaginal Research: a Comparative Review.Reprod Sci. 2021 Jun;28(6):1759-1773. doi: 10.1007/s43032-021-00529-y. Epub 2021 Apr 6. Reprod Sci. 2021. PMID: 33825165 Free PMC article. Review.
-
Fertility preservation in patients with uterus didelphys and endometrial carcinoma: a case report.BMC Womens Health. 2021 Aug 28;21(1):319. doi: 10.1186/s12905-021-01455-6. BMC Womens Health. 2021. PMID: 34454503 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

