Apolipoprotein L1 confers pH-switchable ion permeability to phospholipid vesicles
- PMID: 28918394
- PMCID: PMC5672055
- DOI: 10.1074/jbc.M117.813444
Apolipoprotein L1 confers pH-switchable ion permeability to phospholipid vesicles
Abstract
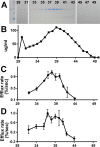
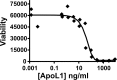
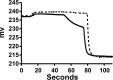
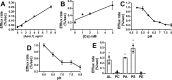
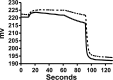
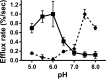
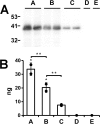
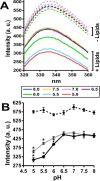
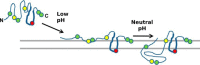
Apolipoprotein L1 (ApoL1) is a human serum protein conferring resistance to African trypanosomes, and certain ApoL1 variants increase susceptibility to some progressive kidney diseases. ApoL1 has been hypothesized to function like a pore-forming colicin and has been reported to have permeability effects on both intracellular and plasma membranes. Here, to gain insight into how ApoL1 may function in vivo, we used vesicle-based ion permeability, direct membrane association, and intrinsic fluorescence to study the activities of purified recombinant ApoL1. We found that ApoL1 confers chloride-selective permeability to preformed phospholipid vesicles and that this selectivity is strongly pH-sensitive, with maximal activity at pH 5 and little activity above pH 7. When ApoL1 and lipid were allowed to interact at low pH and were then brought to neutral pH, chloride permeability was suppressed, and potassium permeability was activated. Both chloride and potassium permeability linearly correlated with the mass of ApoL1 in the reaction mixture, and both exhibited lipid selectivity, requiring the presence of negatively charged lipids for activity. Potassium, but not chloride, permease activity required the presence of calcium ions in both the association and activation steps. Direct assessment of ApoL1-lipid associations confirmed that ApoL1 stably associates with phospholipid vesicles, requiring low pH and the presence of negatively charged phospholipids for maximal binding. Intrinsic fluorescence of ApoL1 supported the presence of a significant structural transition when ApoL1 is mixed with lipids at low pH. This pH-switchable ion-selective permeability may explain the different effects of ApoL1 reported in intracellular and plasma membrane environments.
Keywords: ApoL1; apolipoprotein L1; chloride channel; membrane transporter reconstitution; nephrology; phospholipid vesicle; potassium channel.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health
Figures
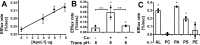

References
-
- Genovese G., Friedman D. J., Ross M. D., Lecordier L., Uzureau P., Freedman B. I., Bowden D. W., Langefeld C. D., Oleksyk T. K., Uscinski Knob A. L., Bernhardy A. J., Hicks P. J., Nelson G. W., Vanhollebeke B., Winkler C. A., et al. (2010) Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science 329, 841–845 - PMC - PubMed
-
- Parsa A., Kao W. H., Xie D., Astor B. C., Li M., Hsu C. Y., Feldman H. I., Parekh R. S., Kusek J. W., Greene T. H., Fink J. C., Anderson A. H., Choi M. J., Wright J. T. Jr., Lash J. P., et al. (2013) APOL1 risk variants, race, and progression of chronic kidney disease. N. Engl. J. Med. 369, 2183–2196 - PMC - PubMed
-
- Pays E., and Vanhollebeke B. (2008) Mutual self-defence: the trypanolytic factor story. Microbes Infect. 10, 985–989 - PubMed
-
- Vanhamme L., Paturiaux-Hanocq F., Poelvoorde P., Nolan D. P., Lins L., Van Den Abbeele J., Pays A., Tebabi P., Van Xong H., Jacquet A., Moguilevsky N., Dieu M., Kane J. P., De Baetselier P., Brasseur R., and Pays E. (2003) Apolipoprotein L-I is the trypanosome lytic factor of human serum. Nature 422, 83–87 - PubMed
-
- Pérez-Morga D., Vanhollebeke B., Paturiaux-Hanocq F., Nolan D. P., Lins L., Homblé F., Vanhamme L., Tebabi P., Pays A., Poelvoorde P., Jacquet A., Brasseur R., and Pays E. (2005) Apolipoprotein L-I promotes trypanosome lysis by forming pores in lysosomal membranes. Science 309, 469–472 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

