Macrophage activation-like syndrome: an immunological entity associated with rapid progression to death in sepsis
- PMID: 28918754
- PMCID: PMC5603161
- DOI: 10.1186/s12916-017-0930-5
Macrophage activation-like syndrome: an immunological entity associated with rapid progression to death in sepsis
Abstract
Background: A subanalysis of a randomized clinical trial indicated sepsis survival benefit from interleukin (IL)-1 blockade in patients with features of the macrophage activation-like syndrome (MALS). This study aimed to investigate the frequency of MALS and to develop a biomarker of diagnosis and prognosis.
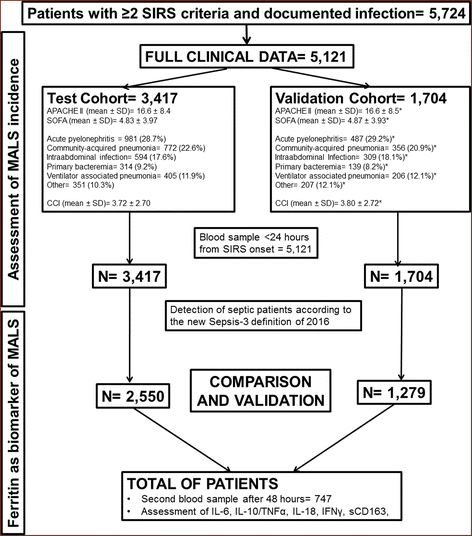
Methods: Patients with infections and systemic inflammatory response syndrome were assigned to one test cohort (n = 3417) and a validation cohort (n = 1704). MALS was diagnosed for patients scoring positive either for the hemophagocytic syndrome score and/or having both hepatobiliary dysfunction and disseminated intravascular coagulation. Logistic regression analysis was used to estimate the predictive value of MALS for 10-day mortality in both cohorts. Ferritin, sCD163, IL-6, IL-10, IL-18, interferon gamma (IFN-γ), and tumor necrosis factor alpha (TNF-α) were measured in the blood the first 24 h; ferritin measurements were repeated in 747 patients on day 3.
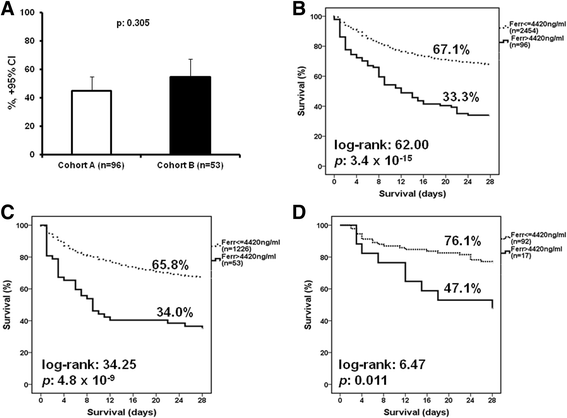
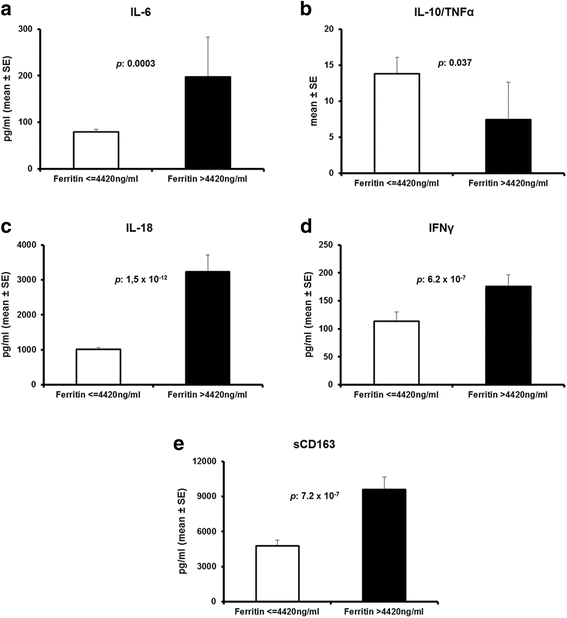
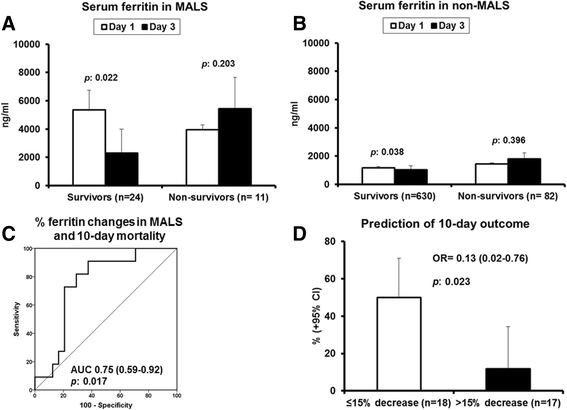
Results: The frequency of MALS was 3.7% and 4.3% in the test and the validation cohort, respectively. In both cohorts, MALS was an independent risk factor for 10-day mortality. A ferritin level above 4420 ng/ml was accompanied by 66.7% and 66% mortality after 28 days, respectively. Ferritin levels above 4420 ng/ml were associated with an increase of IL-6, IL-18, INF-γ, and sCD163 and a decreased IL-10/TNF-α ratio, indicating predominance of pro-inflammatory phenomena. Any less than 15% decrease of ferritin on day 3 was associated with more than 90% sensitivity for unfavorable outcome after 10 days. This high mortality risk was also validated in an independent Swedish cohort (n = 109).
Conclusions: MALS is an independent life-threatening entity in sepsis. Ferritin measurements can provide early diagnosis of MALS and may allow for specific treatment.
Keywords: Ferritin; Interleukin-18; Macrophages; Outcome; Sepsis.
Conflict of interest statement
Ethics approval and consent to participate
The protocol was approved by the following ethics committees:
• Ethics Committee of “Alexandra” General Hospital of Athens
• Ethics Committee of 251 Air Force General Hospital of Athens
• Ethics Committee of Attikon University General Hospital of Athens
• Ethics Committee of Asklipieion General Hospital of Voula, Territory of Athens
• Ethics Committee of “Center for Trauma Resuscitation- KAT” General Hospital of Athens
• Ethics Committee of “Evangelismos” General Hospital of Athens
• Ethics Committee of “Evgenideio” Hospital of Athens
• Ethics Committee of “Hippokrateio” General Hospital of Athens
• Ethics Committee of “Hygeia” General Hospital of Athens
• Ethics Committee of “G. Gennimatas” General Hospital of Athens
• Ethics Committee of “Laikon” General Hospital of Athens
• Ethics Committee of “Konstantopouleio-Aghia Olga” General Hospital of Athens
• Ethics Committee of “Korgialeneion-Benakion” General Hospital of Athens
• Ethics Committee of “Sismanogleion” General Hospital of Athens
• Ethics Committee of “Sotiria” Athens General Hospital
• Ethics Committee of “Thriasio” Elefsis General Hospital Territory of Athens
• Ethics Committee of “Aghios Dimitrios” General Hospital of Thessaloniki
• Ethics Committee of “G. Gennimatas” General Hospital of Thessaloniki
• Ethics Committee of “Aghios Pavlos” General Hospital of Thessaloniki
• Ethics Committee of “Theagenio” Hospital of Thessaloniki
• Ethics Committee of “Metaxa” Hospital of Piraeus
• Ethics Committee of “Tzaneio” General Hospital of Piraeus
• Ethics Committee of University General Hospital of Alexandroupolis
• Ethics Committee of General Hospital of Argos
• Ethics Committee of General Hospital of Arta
• Ethics Committee of General Hospital of Chios
• Ethics Committee of University General Hospital of Ioannina
• Ethics Committee of General Hospital of Karditsa
• Ethics Committee of General Hospital of Korinthos
• Ethics Committee of General Hospital of Lamia
• Ethics Committee of University General Hospital of Larissa
• Ethics Committee of General Hospital of Nicosia
• Ethics Committee of General Hospital of Nafplion
• Ethics Committee of University General Hospital of Patras
• Ethics Committee of General Hospital of Ptolemaida
• Ethics Committee of General Hospital of Sparti
• Ethics Committee of General Hospital of Trikala
• Ethics Committee of General Hospital of Zakynthos
The patients were enrolled after they provided written consent or their legal representative provided it in the case of patients unable to consent.
Competing interests
EJGB has received honoraria (paid to the University of Athens) from AbbVie, Biotest, Brahms GmbH, and The Medicines Company; has received compensation as a consultant for Astellas Greece and for XBiotech (paid to the University of Athens); and has received independent educational grants (paid to the University of Athens) from AbbVie and Sanofi. He is funded by the FrameWork 7 program HemoSpec and by the Horizon 2020 Marie Curie project European Sepsis Academy (granted to the University of Athens). The other authors do not report any competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


References
-
- Opal SM, Fisher CJ, Jr, Dhainaut JF, Vincent JL, Brase R, Lowry SF, et al. Confirmatory interleukin-1 receptor antagonist trial in severe sepsis: a phase III, randomized, double-blind, placebo-controlled, multicenter trial. Crit Care Med. 1997;25:1115–24. doi: 10.1097/00003246-199707000-00010. - DOI - PubMed
-
- Shakoory B, Carcillo JA, Chatham WW, Amdur RL, Zhao H, Dinarello CA, et al. Interleukin-1 receptor blockade is associated with reduced mortality in sepsis patients with features of macrophage activation syndrome: reanalysis of a prior phase III trial. Crit Care Med. 2016;44:275–81. doi: 10.1097/CCM.0000000000001402. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

