Rates of hippocampal atrophy and presence of post-mortem TDP-43 in patients with Alzheimer's disease: a longitudinal retrospective study
- PMID: 28919059
- PMCID: PMC5646369
- DOI: 10.1016/S1474-4422(17)30284-3
Rates of hippocampal atrophy and presence of post-mortem TDP-43 in patients with Alzheimer's disease: a longitudinal retrospective study
Abstract
Background: Post-mortem studies have not identified an association between β-amyloid or tau and rates of hippocampal atrophy in patients with Alzheimer's disease. TAR DNA binding protein 43 (TDP-43) is another protein linked to Alzheimer's disease. We aimed to investigate whether hippocampal TDP-43 is associated with increased rates of hippocampal atrophy.
Methods: In this longitudinal retrospective study, we analysed post-mortem brain tissue of all individuals with an Alzheimer's disease spectrum pathological diagnosis who had antemortem head MRI scans between Jan 1, 1999, and Dec 31, 2012, and who had been recruited into the Mayo Clinic Alzheimer's Disease Research Center, Mayo Clinic Alzheimer's Disease Patient Registry, or the Mayo Clinic Study of Aging. We did TDP-43 immunohistochemistry and classified individuals as follows: no TDP-43 in the amygdala or hippocampus; TDP-43 restricted to the amygdala; and TDP-43 spreading into the hippocampus. Each individual was also assigned a neurofibrillary tangle stage (B1-B3), relating to the likelihood of having Alzheimer's disease. We used longitudinal FreeSurfer software and tensor-based morphometry with symmetric normalisation to calculate hippocampal volume on all serial MRI scans and used linear mixed-effects regression models to estimate associations between TDP-43 and rate of hippocampal atrophy and to assess the trajectory of TDP-43-associated atrophy.
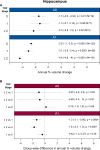
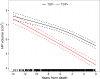
Findings: We identified 298 individuals meeting the inclusion criteria, with 816 usable MRI scans (spanning 1·0-11·2 years of the disease) available for analysis. 141 individuals showed no TDP-43 in the amygdala or hippocampus, 33 had TDP-43 restricted to the amygdala, and 124 had TDP-43 in the hippocampus. Among individuals with a high likelihood of having Alzheimer's disease (neurofibrillary tangle stage B3; n=205), those with hippocampal TDP-43 had faster rates of hippocampal atrophy (n=103, annual volume change -4·39%, 95% CI -4·82 to -3·95; p<0·0001) than did those with amygdala-only TDP-43 (n=20, -3·29%, -4·11 to -2·46; p<0·0001; difference -1·10%, 95% CI -2·02 to -0·19; p=0·02) and those without TDP-43 (n=82, -3·11%, -3·54 to -2·68; p<0·0001; difference -1·28%, -1·88 to -0·67; p<0·0001). Among individuals with an intermediate likelihood of having Alzheimer's disease (neurofibrillary tangle stage B2; n=56), those with hippocampal TDP-43 had faster rates of hippocampal atrophy (n=17, annual volume change -4·05%, 95% CI -5·09 to -2·99; p<0·0001) than did those with amygdala-only TDP-43 (n=6, -1·78%, -3·04 to -0·55; p=0·004; difference -2·27%, 95% CI -3·79 to -0·67; p=0·006) and those without TDP-43 (n=33, -1·63%, -2·43 to -0·83; p=0·0002; difference -2·43%, -3·66 to -1·18; p=0·0002). Hippocampal TDP-43 was not associated with the rate of hippocampal atrophy in individuals with a low likelihood of having Alzheimer's disease (neurofibrillary tangle stage B1; n=37). The trajectory analysis suggested that increased rates of TDP-43-associated hippocampal atrophy might occur at least 10 years before death. Results were similar for FreeSurfer and tensor-based morphometry.
Interpretation: TDP-43 should be considered as a potential factor related to increased rates of hippocampal atrophy in patients with Alzheimer's disease. Given the importance of hippocampal atrophy in Alzheimer's disease, it is imperative that techniques are developed for detection of TDP-43 in vivo.
Funding: US National Institute on Aging (National Institutes of Health).
Copyright © 2017 Elsevier Ltd. All rights reserved.
Figures


Comment in
-
Pathology and hippocampal atrophy in Alzheimer's disease.Lancet Neurol. 2017 Nov;16(11):862-864. doi: 10.1016/S1474-4422(17)30343-5. Lancet Neurol. 2017. PMID: 29029840 No abstract available.
References
-
- Silbert LC, Quinn JF, Moore MM, et al. Changes in premorbid brain volume predict Alzheimer's disease pathology. Neurology. 2003;61(4):487–92. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

