Phospho-Regulation of Soma-to-Axon Transcytosis of Neurotrophin Receptors
- PMID: 28919207
- PMCID: PMC5614868
- DOI: 10.1016/j.devcel.2017.08.009
Phospho-Regulation of Soma-to-Axon Transcytosis of Neurotrophin Receptors
Abstract
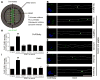
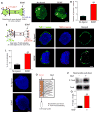
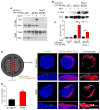
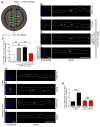
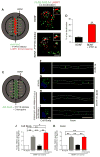
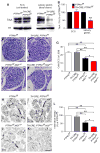
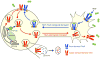
Axonal targeting of signaling receptors is essential for neuronal responses to extracellular cues. Here, we report that retrograde signaling by target-derived nerve growth factor (NGF) is necessary for soma-to-axon transcytosis of TrkA receptors in sympathetic neurons, and we define the molecular underpinnings of this positive feedback regulation that enhances neuronal sensitivity to trophic factors. Activated TrkA receptors are retrogradely transported in signaling endosomes from distal axons to cell bodies, where they are inserted on soma surfaces and promote phosphorylation of resident naive receptors, resulting in their internalization. Endocytosed TrkA receptors are then dephosphorylated by PTP1B, an ER-resident protein tyrosine phosphatase, prior to axonal transport. PTP1B inactivation prevents TrkA exit from soma and causes receptor degradation, suggesting a "gatekeeper" mechanism that ensures targeting of inactive receptors to axons to engage with ligand. In mice, PTP1B deletion reduces axonal TrkA levels and attenuates neuron survival and target innervation under limiting NGF (NGF+/-) conditions.
Keywords: ER; PTP1B; TrkA signaling endosomes; axon transport; neurotrophins; protein tyrosine phosphatase; sympathetic neuron development; transcytosis.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures
Comment in
-
TrkA Bumps into Its Future Self.Dev Cell. 2017 Sep 25;42(6):557-558. doi: 10.1016/j.devcel.2017.09.002. Dev Cell. 2017. PMID: 28950095
Similar articles
-
Transcytosis-mediated anterograde transport of TrkA receptors is necessary for sympathetic neuron development and function.Proc Natl Acad Sci U S A. 2023 Feb 7;120(6):e2205426120. doi: 10.1073/pnas.2205426120. Epub 2023 Feb 2. Proc Natl Acad Sci U S A. 2023. PMID: 36730190 Free PMC article.
-
Axonal targeting of Trk receptors via transcytosis regulates sensitivity to neurotrophin responses.J Neurosci. 2009 Sep 16;29(37):11674-85. doi: 10.1523/JNEUROSCI.1542-09.2009. J Neurosci. 2009. PMID: 19759314 Free PMC article.
-
Characterization of an NGF-P-TrkA retrograde-signaling complex and age-dependent regulation of TrkA phosphorylation in sympathetic neurons.J Neurosci. 1999 Oct 1;19(19):8207-18. doi: 10.1523/JNEUROSCI.19-19-08207.1999. J Neurosci. 1999. PMID: 10493722 Free PMC article.
-
Biogenesis and function of the NGF/TrkA signaling endosome.Int Rev Cell Mol Biol. 2015;314:239-57. doi: 10.1016/bs.ircmb.2014.10.002. Epub 2014 Nov 18. Int Rev Cell Mol Biol. 2015. PMID: 25619719 Free PMC article. Review.
-
Retrograde transport of neurotrophins: fact and function.J Neurobiol. 2004 Feb 5;58(2):217-29. doi: 10.1002/neu.10322. J Neurobiol. 2004. PMID: 14704954 Review.
Cited by
-
Recent advances in understanding context-dependent mechanisms controlling neurotrophin signaling and function.F1000Res. 2019 Sep 19;8:F1000 Faculty Rev-1658. doi: 10.12688/f1000research.19174.1. eCollection 2019. F1000Res. 2019. PMID: 31583078 Free PMC article. Review.
-
Spatial regulation of endosomes in growing dendrites.Dev Biol. 2022 Jun;486:5-14. doi: 10.1016/j.ydbio.2022.03.004. Epub 2022 Mar 17. Dev Biol. 2022. PMID: 35306006 Free PMC article. Review.
-
ER and Golgi trafficking in axons, dendrites, and glial processes.Curr Opin Cell Biol. 2022 Oct;78:102119. doi: 10.1016/j.ceb.2022.102119. Epub 2022 Aug 11. Curr Opin Cell Biol. 2022. PMID: 35964523 Free PMC article. Review.
-
Effects of Reactive Oxygen and Nitrogen Species on TrkA Expression and Signalling: Implications for proNGF in Aging and Alzheimer's Disease.Cells. 2021 Aug 4;10(8):1983. doi: 10.3390/cells10081983. Cells. 2021. PMID: 34440751 Free PMC article. Review.
-
Mechanisms of neurotrophin trafficking via Trk receptors.Mol Cell Neurosci. 2018 Sep;91:25-33. doi: 10.1016/j.mcn.2018.03.013. Epub 2018 Mar 27. Mol Cell Neurosci. 2018. PMID: 29596897 Free PMC article. Review.
References
-
- Arimura N, Kimura T, Nakamuta S, Taya S, Funahashi Y, Hattori A, Shimada A, Menager C, Kawabata S, Fujii K, et al. Anterograde transport of TrkB in axons is mediated by direct interaction with Slp1 and Rab27. Developmental cell. 2009;16:675–686. - PubMed
-
- Bence KK, Delibegovic M, Xue B, Gorgun CZ, Hotamisligil GS, Neel BG, Kahn BB. Neuronal PTP1B regulates body weight, adiposity and leptin action. Nature medicine. 2006;12:917–924. - PubMed
-
- Bhattacharyya A, Watson FL, Pomeroy SL, Zhang YZ, Stiles CD, Segal RA. High-resolution imaging demonstrates dynein-based vesicular transport of activated Trk receptors. Journal of neurobiology. 2002;51:302–312. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

