Trace amine-associated receptor 1 regulation of methamphetamine-induced neurotoxicity
- PMID: 28919515
- PMCID: PMC5683899
- DOI: 10.1016/j.neuro.2017.09.006
Trace amine-associated receptor 1 regulation of methamphetamine-induced neurotoxicity
Abstract
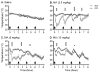
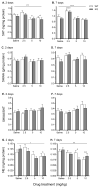
Trace amine-associated receptor 1 (TAAR1) is activated by methamphetamine (MA) and modulates dopaminergic (DA) function. Although DA dysregulation is the hallmark of MA-induced neurotoxicity leading to behavioral and cognitive deficits, the intermediary role of TAAR1 has yet to be characterized. To investigate TAAR1 regulation of MA-induced neurotoxicity, Taar1 transgenic knock-out (KO) and wildtype (WT) mice were administered saline or a neurotoxic regimen of 4 i.p. injections, 2h apart, of MA (2.5, 5, or 10mg/kg). Temperature data were recorded during the treatment day. Additionally, striatal tissue was collected 2 or 7days following MA administration for analysis of DA, 3,4-dihydroxyphenylacetic acid (DOPAC), homovanillic acid (HVA), and tyrosine hydroxylase (TH) levels, as well as glial fibrillary acidic protein (GFAP) expression. MA elicited an acute hypothermic drop in body temperature in Taar1-WT mice, but not in Taar1-KO mice. Two days following treatment, DA and TH levels were lower in Taar1-KO mice compared to Taar1-WT mice, regardless of treatment, and were dose-dependently decreased by MA. GFAP expression was significantly increased by all doses of MA at both time points and greater in Taar1-KO compared to Taar1-WT mice receiving MA 2.5 or 5mg/kg. Seven days later, DA levels were decreased in a similar pattern: DA was significantly lower in Taar1-KO compared to Taar1-WT mice receiving MA 2.5 or 5mg/kg. TH levels were uniformly decreased by MA, regardless of genotype. These results indicate that activation of TAAR1 potentiates MA-induced hypothermia and TAAR1 confers sustained neuroprotection dependent on its thermoregulatory effects.
Keywords: Dopamine; GFAP; Methamphetamine; Neurotoxicity; TAAR1; Temperature.
Published by Elsevier B.V.
Conflict of interest statement
The authors do not have any conflicts of interest to declare.
Figures

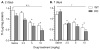
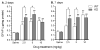
References
-
- Achat-Mendes C, Lynch LJ, Sullivan KA, Vallender EJ, Miller GM. Augmentation of methamphetamine-induced behaviors in transgenic mice lacking the trace amine-associated receptor 1. Pharmacol Biochem Behav. 2012;101:201–207. doi: 10.1016/j.pbb.2011.10.025. S0091-3057(11)00354-6 [pii] - DOI - PMC - PubMed
-
- Albers DS, Sonsalla PK. Methamphetamine-induced hyperthermia and dopaminergic neurotoxicity in mice: pharmacological profile of protective and nonprotective agents. J Pharmacol Exp Ther. 1995;275:1104–1114. - PubMed
-
- Ali SF, Newport GD, Slikker W., Jr Methamphetamine-induced dopaminergic toxicity in mice. Role of environmental temperature and pharmacological agents. Ann N Y Acad Sci. 1996;801:187–198. - PubMed
-
- Alvarsson A, Zhang X, Stan TL, Schintu N, Kadkhodaei B, Millan MJ, Perlmann T, Svenningsson P. Modulation by Trace Amine-Associated Receptor 1 of Experimental Parkinsonism, l-DOPA Responsivity, and Glutamatergic Neurotransmission. J Neurosci. 2015;35:14057–14069. doi: 10.1523/JNEUROSCI.1312-15.2015. 35/41/14057 [pii] - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

