Micro-invasive glaucoma surgery (MIGS): a review of surgical procedures using stents
- PMID: 28919702
- PMCID: PMC5587165
- DOI: 10.2147/OPTH.S135316
Micro-invasive glaucoma surgery (MIGS): a review of surgical procedures using stents
Erratum in
-
Erratum: Micro-invasive glaucoma surgery (MIGS): a review of surgical procedures using stents [Corrigendum].Clin Ophthalmol. 2018 Feb 5;12:287. doi: 10.2147/OPTH.S160409. eCollection 2018. Clin Ophthalmol. 2018. PMID: 29440871 Free PMC article.
Abstract
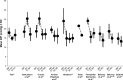
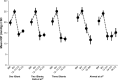
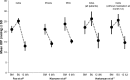
Over the last decade several novel surgical treatment options and devices for glaucoma have been developed. All these developments aim to cause as little trauma as possible to the eye, to safely, effectively, and sustainably reduce intraocular pressure (IOP), to produce reproducible results, and to be easy to adopt. The term "micro-invasive glaucoma surgery (MIGS)" was used for summarizing all these procedures. Currently MIGS is gaining more and more interest and popularity. The possible reduction of the number of glaucoma medications, the ab interno approach without damaging the conjunctival tissue, and the probably safer procedures compared to incisional surgical methods may explain the increased interest in MIGS. The use of glaucoma drainage implants for lowering IOP in difficult-to-treat patients has been established for a long time, however, a variety of new glaucoma micro-stents are being manufactured by using various materials and are available to increase aqueous outflow via different pathways. This review summarizes published results of randomized clinical studies and extensive case report series on these devices, including Schlemm's canal stents (iStent®, iStent® inject, Hydrus), suprachoroidal stents (CyPass®, iStent® Supra), and subconjunctival stents (XEN). The article summarizes the findings of published material on efficacy and safety for each of these approaches.
Keywords: CyPass; Hydrus; MIGS; XEN; glaucoma; iStent; iStent inject; micro-invasive glaucoma surgery.
Conflict of interest statement
Disclosure Medical writing of this review was done by eyecons (F Kimmich) with financial support from Glaukos Inc. San Clemente, USA. C Erb and A Jünemann are speakers for Glaukos Inc. L Pillunat has no conflicts of interest in this work.
Figures




References
-
- Chauhan BC, Mikelberg FS, Balaszi AG, LeBlanc RP, Lesk MR, Trope GE, Canadian Glaucoma Study Group Canadian Glaucoma Study: 2. risk factors for the progression of open-angle glaucoma. Arch Ophthalmol. 2008;126(8):1030–1036. - PubMed
-
- The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. The AGIS Investigators. Am J Ophthalmol. 2000;130(4):429–440. No authors listed. - PubMed
-
- Heijl A, Leske MC, Bengtsson B, Hyman L, Bengtsson B, Hussein M, Early Manifest Glaucoma Trial Group Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002;120(10):1268–1279. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

