Zinc oxide nanoparticles induce apoptosis and autophagy in human ovarian cancer cells
- PMID: 28919752
- PMCID: PMC5592910
- DOI: 10.2147/IJN.S140071
Zinc oxide nanoparticles induce apoptosis and autophagy in human ovarian cancer cells
Abstract
Background: Zinc oxide nanoparticles (ZnO NPs) are frequently used in industrial products such as paint, surface coating, and cosmetics, and recently, they have been explored in biologic and biomedical applications. Therefore, this study was undertaken to investigate the effect of ZnO NPs on cytotoxicity, apoptosis, and autophagy in human ovarian cancer cells (SKOV3).
Methods: ZnO NPs with a crystalline size of 20 nm were characterized with various analytical techniques, including ultraviolet-visible spectroscopy, X-ray diffraction, transmission electron microscopy, Fourier transform infrared spectroscopy, and atomic force microscopy. The cytotoxicity, apoptosis, and autophagy were examined using a series of cellular assays.
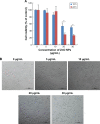
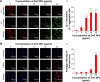
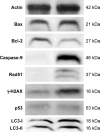
Results: Exposure of cells to ZnO NPs resulted in a dose-dependent loss of cell viability, and the characteristic apoptotic features such as rounding and loss of adherence, enhanced reactive oxygen species generation, and loss of mitochondrial membrane potential were observed in the ZnO NP-treated cells. Furthermore, the cells treated with ZnO NPs showed significant double-strand DNA breaks, which are gained evidences from significant number of γ-H2AX and Rad51 expressed cells. ZnO NP-treated cells showed upregulation of p53 and LC3, indicating that ZnO NPs are able to upregulate apoptosis and autophagy. Finally, the Western blot analysis revealed upregulation of Bax, caspase-9, Rad51, γ-H2AX, p53, and LC3 and downregulation of Bcl-2.
Conclusion: The study findings demonstrated that the ZnO NPs are able to induce significant cytotoxicity, apoptosis, and autophagy in human ovarian cells through reactive oxygen species generation and oxidative stress. Therefore, this study suggests that ZnO NPs are suitable and inherent anticancer agents due to their several favorable characteristic features including favorable band gap, electrostatic charge, surface chemistry, and potentiation of redox cycling cascades.
Keywords: DNA fragmentation; apoptosis; autophagy; human ovarian cancer cells SKOV3; mitochondrial membrane potential; zinc oxide nanoparticles.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures




Similar articles
-
Cytotoxic effects of ZnO nanoparticles on mouse testicular cells.Int J Nanomedicine. 2016 Oct 11;11:5187-5203. doi: 10.2147/IJN.S111447. eCollection 2016. Int J Nanomedicine. 2016. PMID: 27785022 Free PMC article.
-
Zinc oxide nanoparticles selectively induce apoptosis in human cancer cells through reactive oxygen species.Int J Nanomedicine. 2012;7:845-57. doi: 10.2147/IJN.S29129. Epub 2012 Feb 21. Int J Nanomedicine. 2012. PMID: 22393286 Free PMC article.
-
Zinc oxide nanoparticles induce lipoxygenase-mediated apoptosis and necrosis in human neuroblastoma SH-SY5Y cells.Neurochem Int. 2015 Nov;90:204-14. doi: 10.1016/j.neuint.2015.09.002. Epub 2015 Sep 11. Neurochem Int. 2015. PMID: 26364578
-
Zinc oxide nanoparticles: Synthesis, antiseptic activity and toxicity mechanism.Adv Colloid Interface Sci. 2017 Nov;249:37-52. doi: 10.1016/j.cis.2017.07.033. Epub 2017 Aug 26. Adv Colloid Interface Sci. 2017. PMID: 28923702 Review.
-
ZnO nanomaterials target mitochondrial apoptosis and mitochondrial autophagy pathways in cancer cells.Cell Biochem Funct. 2024 Jan;42(1):e3909. doi: 10.1002/cbf.3909. Cell Biochem Funct. 2024. PMID: 38269499 Review.
Cited by
-
Green synthesis of zinc oxide nanoparticles using aqueous extract of shilajit and their anticancer activity against HeLa cells.Sci Rep. 2024 Jan 25;14(1):2204. doi: 10.1038/s41598-024-52217-x. Sci Rep. 2024. PMID: 38273022 Free PMC article.
-
The Role of Dietary Polyphenols in Pregnancy and Pregnancy-Related Disorders.Nutrients. 2022 Dec 9;14(24):5246. doi: 10.3390/nu14245246. Nutrients. 2022. PMID: 36558404 Free PMC article. Review.
-
Zinc oxide nanoparticles induce toxicity in CAL 27 oral cancer cell lines by activating PINK1/Parkin-mediated mitophagy.Int J Nanomedicine. 2018 Jun 20;13:3441-3450. doi: 10.2147/IJN.S165699. eCollection 2018. Int J Nanomedicine. 2018. PMID: 29950828 Free PMC article.
-
Functionalized and Nonfunctionalized Nanosystems for Mitochondrial Drug Delivery with Metallic Nanoparticles.Molecules. 2023 Jun 12;28(12):4701. doi: 10.3390/molecules28124701. Molecules. 2023. PMID: 37375256 Free PMC article. Review.
-
SnO2-Doped ZnO/Reduced Graphene Oxide Nanocomposites: Synthesis, Characterization, and Improved Anticancer Activity via Oxidative Stress Pathway.Int J Nanomedicine. 2021 Jan 8;16:89-104. doi: 10.2147/IJN.S285392. eCollection 2021. Int J Nanomedicine. 2021. PMID: 33447029 Free PMC article.
References
-
- Zhao J, Castranova V. Toxicology of nanomaterials used in nanomedicine. J Toxicol Environ Health B Crit Rev. 2011;14(8):593–632. - PubMed
-
- Becheri A, Dürr M, Nostro PL, Baglioni P. Synthesis and characterization of zinc oxide nanoparticles: application to textiles as UV–absorbers. J Nanopart Res. 2008;10(4):679–689.
-
- Wang H, Wick RL, Xing B. Toxicity of nanoparticulate and bulk ZnO, Al2O3 and TiO2 to the nematode Caenorhabditis elegans. Environ Pollut. 2009;157(4):1171–1177. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

