Response to duloxetine in chronic low back pain: exploratory post hoc analysis of a Japanese Phase III randomized study
- PMID: 28919811
- PMCID: PMC5590685
- DOI: 10.2147/JPR.S138172
Response to duloxetine in chronic low back pain: exploratory post hoc analysis of a Japanese Phase III randomized study
Abstract
Purpose: Duloxetine is efficacious for chronic low back pain (CLBP). This post hoc analysis of a Japanese randomized, placebo-controlled trial (ClinicalTrials.gov, NCT01855919) assessed whether patients with CLBP with early pain reduction or treatment-related adverse events of special interest (TR-AESIs; nausea, somnolence, constipation) have enhanced responses to duloxetine.
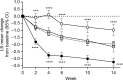
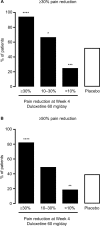
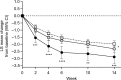
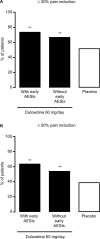
Patients and methods: Patients (N = 456) with CLBP for ≥6 months and Brief Pain Inventory (BPI) average pain severity score of ≥4 were randomized (1:1) to duloxetine 60 mg/day or placebo for 14 weeks. Primary outcome was change from baseline in BPI average pain severity score (pain reduction). Subgroup analyses included early pain reduction (≥30%, 10%-30%, or <10% at Week 4) and early TR-AESIs (with or without TR-AESIs by Week 2). Measures included changes from baseline in BPI average pain severity score and BPI Interference scores (quality of life; QOL), and response rate (≥30% or ≥50% pain reduction at Week 14).
Results: Patients with ≥30% early pain reduction (n = 108) or early TR-AESIs (n = 50) had significantly greater improvements in pain and QOL than placebo-treated patients (n = 226), whereas patients with 10%-30% (n = 63) or <10% (n = 48) pain reduction did not; patients without early TR-AESIs (n = 180) had significant improvements in pain at Week 14. Response rates (≥30%/≥50% pain reduction) were 94.4%/82.4%, 66.7%/49.2%, and 25.0%/18.8% for patients with ≥30%, 10%-30%, and <10% early pain reduction, respectively, 74.0%/64.0% for patients with early TR-AESIs, 67.2%/54.4% for patients without early TR-AESIs, and 52.2%/39.4% for placebo.
Conclusion: Early pain reduction or TR-AESIs may predict which CLBP patients are most likely to respond to duloxetine with improvements in pain and QOL.
Keywords: Brief Pain Inventory; adverse events; duloxetine; low back pain; quality of life; responder.
Conflict of interest statement
Disclosure TT, NI, MI, and TO are employees and minor stock holders of Shionogi & Co. Ltd. SK has received consulting fees and honoraria from Eli Lilly Japan K.K. and Shionogi & Co. Ltd, and has received research grants from Shionogi & Co. Ltd. The authors report no other conflicts of interest in this work.
Figures



References
-
- Hoy D, Brooks P, Blyth F, Buchbinder R. The epidemiology of low back pain. Best Pract Res Clin Rheumatol. 2010;24(6):769–781. - PubMed
-
- Institute of Medicine (US) Committee on Advancing Pain Research, Care, and Education . Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington (DC): National Academies Press; 2011. - PubMed
-
- Webster LR, Markman J. Medical management of chronic low back pain: efficacy and outcomes. Neuromodulation. 2014;17(Suppl 2):18–23. - PubMed
-
- Dagenais S, Caro J, Haldeman S. A systematic review of low back pain cost of illness studies in the United States and internationally. Spine J. 2008;8(1):8–20. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

