Umbilical cord blood CD34+ progenitor-derived NK cells efficiently kill ovarian cancer spheroids and intraperitoneal tumors in NOD/SCID/IL2Rgnull mice
- PMID: 28919991
- PMCID: PMC5593716
- DOI: 10.1080/2162402X.2017.1320630
Umbilical cord blood CD34+ progenitor-derived NK cells efficiently kill ovarian cancer spheroids and intraperitoneal tumors in NOD/SCID/IL2Rgnull mice
Abstract
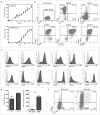
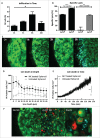
Adoptive transfer of allogeneic natural killer (NK) cells is an attractive therapy approach against ovarian carcinoma. Here, we evaluated the potency of highly active NK cells derived from human CD34+ haematopoietic stem and progenitor cells (HSPC) to infiltrate and mediate killing of human ovarian cancer spheroids using an in vivo-like model system and mouse xenograft model. These CD56+Perforin+ HSPC-NK cells were generated under stroma-free conditions in the presence of StemRegenin-1, IL-15, and IL-12, and exerted efficient cytolytic activity and IFNγ production toward ovarian cancer monolayer cultures. Live-imaging confocal microscopy demonstrated that these HSPC-NK cells actively migrate, infiltrate, and mediate tumor cell killing in a three-dimensional multicellular ovarian cancer spheroid. Infiltration of up to 30% of total HSPC-NK cells within 8 h resulted in robust tumor spheroid destruction. Furthermore, intraperitoneal HSPC-NK cell infusions in NOD/SCID-IL2Rγnull (NSG) mice bearing ovarian carcinoma significantly reduced tumor progression. These findings demonstrate that highly functional HSPC-NK cells efficiently destruct ovarian carcinoma spheroids in vitro and kill intraperitoneal ovarian tumors in vivo, providing great promise for effective immunotherapy through intraperitoneal HSPC-NK cell adoptive transfer in ovarian carcinoma patients.
Keywords: Adoptive immunotherapy; NK cells; mouse ovarian cancer xenograft; ovarian cancer; tumor spheroid infiltration.
Figures



References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015; 65(1):5-29; PMID:25559415; https://doi.org/10.3322/caac.21254 - DOI - PubMed
-
- Geller MA, Knorr DA, Hermanson DA, Pribyl L, Bendzick L, McCullar V, Miller JS, Kaufman DS. Intraperitoneal delivery of human natural killer cells for treatment of ovarian cancer in a mouse xenograft model. Cytotherapy 2013; 15(10):1297-306; PMID:23993303; https://doi.org/10.1016/j.jcyt.2013.05.022 - DOI - PMC - PubMed
-
- Hermanson DL, Bendzick L, Pribyl L, McCullar V, Vogel RI, Miller JS, Geller MA, Kaufman DS. Induced pluripotent stem cell-derived natural killer cells for treatment of ovarian cancer. Stem Cells 2016; 34(1):93-101; PMID:26503833; https://doi.org/10.1002/stem.2230 - DOI - PMC - PubMed
-
- Davis ZB, Felices M, Verneris MR, Miller JS. Natural killer cell adoptive transfer therapy: Exploiting the first line of defense against cancer. Cancer J 2015; 21(6):486-491; PMID:26588681; https://doi.org/10.1097/PPO.0000000000000156 - DOI - PMC - PubMed
-
- Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK, McKenna D, Le C, Defor TE, Burns LJ et al.. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood 2005; 105(8):3051-7; PMID:15632206; https://doi.org/10.1182/blood-2004-07-2974 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
