Towards Treatment of Stargardt Disease: Workshop Organized and Sponsored by the Foundation Fighting Blindness
- PMID: 28920007
- PMCID: PMC5599228
- DOI: 10.1167/tvst.6.5.6
Towards Treatment of Stargardt Disease: Workshop Organized and Sponsored by the Foundation Fighting Blindness
Abstract
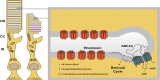
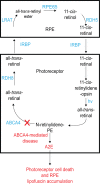
Accumulation of fluorescent metabolic byproducts of the visual (retinoid) cycle is associated with photoreceptor and retinal pigment epithelial cell death in both Stargardt disease and atrophic (nonneovascular) age-related macular degeneration (AMD). As a consequence of this observation, small molecular inhibitors of enzymes in the visual cycle were recently tested in clinical trials as a strategy to protect the retina and retinal pigment epithelium in patients with atrophic AMD. To address the clinical translational needs for therapies aimed at both diseases, a workshop organized by the Foundation Fighting Blindness was hosted by the Department of Pharmacology at Case Western Reserve University on February 17, 2017, at the Tinkham Veale University Center, Cleveland, OH, USA. Invited speakers highlighted recent advances in the understanding of the pathophysiology of Stargardt disease, in terms of its clinical characterization and the development of endpoints for clinical trials, and discussed the comparability of therapeutic strategies between atrophic age-related macular degeneration (AMD) and Stargardt disease. Investigators speculated that reducing the concentrations of visual cycle precursor substances and/or their byproducts may provide valid therapeutic options for the treatment of Stargardt disease. Here we review the workshop's presentations in the context of published literature to help shape the aims of ongoing research endeavors and aid the development of therapies for Stargardt disease.
Keywords: A2E, all-trans-retinal; ABCA4; Stargardt disease; age-related macular degeneration; geographic atrophy; lipofuscin.
Figures
Similar articles
-
Systemic administration of the di-apocarotenoid norbixin (BIO201) is neuroprotective, preserves photoreceptor function and inhibits A2E and lipofuscin accumulation in animal models of age-related macular degeneration and Stargardt disease.Aging (Albany NY). 2020 Apr 7;12(7):6151-6171. doi: 10.18632/aging.103014. Epub 2020 Apr 7. Aging (Albany NY). 2020. PMID: 32255762 Free PMC article.
-
A non-retinoid antagonist of retinol-binding protein 4 rescues phenotype in a model of Stargardt disease without inhibiting the visual cycle.J Biol Chem. 2018 Jul 20;293(29):11574-11588. doi: 10.1074/jbc.RA118.002062. Epub 2018 Jun 5. J Biol Chem. 2018. PMID: 29871924 Free PMC article.
-
Dual ABCA4-AAV Vector Treatment Reduces Pathogenic Retinal A2E Accumulation in a Mouse Model of Autosomal Recessive Stargardt Disease.Hum Gene Ther. 2019 Nov;30(11):1361-1370. doi: 10.1089/hum.2019.132. Epub 2019 Sep 30. Hum Gene Ther. 2019. PMID: 31418294 Free PMC article.
-
Defective lipid transport and biosynthesis in recessive and dominant Stargardt macular degeneration.Prog Lipid Res. 2010 Oct;49(4):476-92. doi: 10.1016/j.plipres.2010.07.002. Epub 2010 Jul 13. Prog Lipid Res. 2010. PMID: 20633576 Free PMC article. Review.
-
Lipofuscin and macular degeneration.Nutr Rev. 2003 Oct;61(10):342-6. doi: 10.1301/nr.2003.oct.342-346. Nutr Rev. 2003. PMID: 14604266 Review.
Cited by
-
Preclinical pharmacology of a lipophenol in a mouse model of light-induced retinopathy.Exp Mol Med. 2020 Jul;52(7):1090-1101. doi: 10.1038/s12276-020-0460-7. Epub 2020 Jul 8. Exp Mol Med. 2020. PMID: 32641711 Free PMC article.
-
Systemic administration of the di-apocarotenoid norbixin (BIO201) is neuroprotective, preserves photoreceptor function and inhibits A2E and lipofuscin accumulation in animal models of age-related macular degeneration and Stargardt disease.Aging (Albany NY). 2020 Apr 7;12(7):6151-6171. doi: 10.18632/aging.103014. Epub 2020 Apr 7. Aging (Albany NY). 2020. PMID: 32255762 Free PMC article.
-
Visual cycle modulators versus placebo or observation for the prevention and treatment of geographic atrophy due to age-related macular degeneration.Cochrane Database Syst Rev. 2020 Dec 17;12(12):CD013154. doi: 10.1002/14651858.CD013154.pub2. Cochrane Database Syst Rev. 2020. PMID: 33331670 Free PMC article.
-
Retinal stem cell transplantation: Balancing safety and potential.Prog Retin Eye Res. 2020 Mar;75:100779. doi: 10.1016/j.preteyeres.2019.100779. Epub 2019 Sep 5. Prog Retin Eye Res. 2020. PMID: 31494256 Free PMC article. Review.
-
Correlation of Morphology and Function of Flecks Using Short-Wave Fundus Autofluorescence and Microperimetry in Patients With Stargardt Disease.Transl Vis Sci Technol. 2021 Mar 1;10(3):18. doi: 10.1167/tvst.10.3.18. Transl Vis Sci Technol. 2021. PMID: 34003952 Free PMC article.
References
-
- Allikmets R, Singh N, Sun H,et al. A photoreceptor cell-specific ATP-binding transporter gene (ABCR) is mutated in recessive Stargardt macular dystrophy. Nat Genet. 1997; 15: 236– 246. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

