The IL-1RI Co-Receptor TILRR (FREM1 Isoform 2) Controls Aberrant Inflammatory Responses and Development of Vascular Disease
- PMID: 28920098
- PMCID: PMC5582195
- DOI: 10.1016/j.jacbts.2017.03.014
The IL-1RI Co-Receptor TILRR (FREM1 Isoform 2) Controls Aberrant Inflammatory Responses and Development of Vascular Disease
Abstract
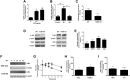
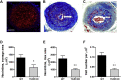
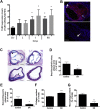
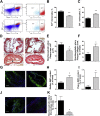
Expression of the interleukin-1 receptor type I (IL-1RI) co-receptor Toll-like and interleukin-1 receptor regulator (TILRR) is significantly increased in blood monocytes following myocardial infarction and in the atherosclerotic plaque, whereas levels in healthy tissue are low. TILRR association with IL-1RI at these sites causes aberrant activation of inflammatory genes, which underlie progression of cardiovascular disease. The authors show that genetic deletion of TILRR or antibody blocking of TILRR function reduces development of atherosclerotic plaques. Lesions exhibit decreased levels of monocytes, with increases in collagen and smooth muscle cells, characteristic features of stable plaques. The results suggest that TILRR may constitute a rational target for site- and signal-specific inhibition of vascular disease.
Keywords: ApoE, apolipoprotein E; DK, double knockout; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; IL, interleukin; IL-1RI; IL-1RI, interleukin-1 receptor type I; IgG, immunoglobulin G; IκBα, inhibitor kappa B alpha; KO, knockout; LDLR–/–, low-density lipoprotein receptor–/–; LPS, lipopolysaccharide; NF-κB; NF-κB, nuclear factor-kappa B; NSTEMI, non–ST-segment elevation myocardial infarction; PBS, phosphate-buffered saline; PCR, polymerase chain reaction; SDS, sodium dodecyl sulfate; STEMI, ST-segment elevation myocardial infarction; TILRR; TILRR, toll-like and interleukin-1 receptor regulator; heparan sulfate proteoglycan; iBALT, inducible bronchus-associated lymphoid tissue; interleukin-1 receptor; qPCR, quantitative polymerase chain reaction.
Figures

References
-
- Gay N.J., Ganloff M. Structure and function of Toll-like receptors and their ligands. Annu Rev Biochem. 2007;76:141–165. - PubMed
-
- Michel T., Reichhart J.M., Hoffmann J.A., Royet J. Drosophila Toll is activated by gram-positive bacteria through a circulating peptideglycan recognition protein. Nature. 2001;414:756–759. - PubMed
-
- Beutler B. Inferences, questions and possibilities in toll-like receptor signalling. Nature. 2004;430:257–263. - PubMed
-
- O'Neill L.A.J., Golenbock D., Bowie A.G. The history of Toll-like receptors - redefining innate immunity. Nat Rev Immunol. 2013;13:453–460. - PubMed
-
- Kirkbride K.C., Ray B.N., Blobe G.C. Cell-surface co-receptors: emerging roles in signaling and human disease. Trends Biochem Sci. 2005;30:611–621. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

