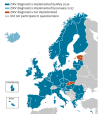
Status, quality and specific needs of Zika virus (ZIKV) diagnostic capacity and capability in National Reference Laboratories for arboviruses in 30 EU/EEA countries, May 2016
- PMID: 28920574
- PMCID: PMC5685210
- DOI: 10.2807/1560-7917.ES.2017.22.36.30609
Status, quality and specific needs of Zika virus (ZIKV) diagnostic capacity and capability in National Reference Laboratories for arboviruses in 30 EU/EEA countries, May 2016
Abstract
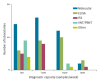
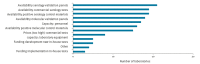
With international travel, Zika virus (ZIKV) is introduced to Europe regularly. A country's ability to robustly detect ZIKV introduction and local transmission is important to minimise the risk for a ZIKV outbreak. Therefore, sufficient expertise and diagnostic capacity and capability are required in European laboratories. To assess the capacity, quality, operational specifics (guidelines and algorithms), technical and interpretation issues and other possible difficulties that were related to ZIKV diagnostics in European countries, a questionnaire was conducted among national reference laboratories in 30 countries in the European Union/European Economic Area (EU/EEA) in May 2016. While the coverage and capacity of ZIKV diagnostics in the EU/EEA national reference laboratories were found to be adequate, the assessment of the quality and needs indicated several crucial points of improvement that will need support at national and EU/EEA level to improve ZIKV preparedness, response and EU/EEA ZIKV surveillance activities.
Keywords: ZIKV; Zika virus; diagnostic; emerging diseases; laboratory preparedness and response; re-emerging diseases; vector-borne infections.
This article is copyright of The Authors, 2017.
Conflict of interest statement
Figures
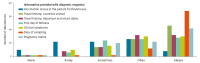
Similar articles
-
Surveillance of Zika virus infection in the EU/EEA, June 2015 to January 2017.Euro Surveill. 2017 Oct;22(41):17-00254. doi: 10.2807/1560-7917.ES.2017.22.41.17-00254. Euro Surveill. 2017. PMID: 29043960 Free PMC article.
-
Variable Sensitivity in Molecular Detection of Zika Virus in European Expert Laboratories: External Quality Assessment, November 2016.J Clin Microbiol. 2017 Nov;55(11):3219-3226. doi: 10.1128/JCM.00987-17. Epub 2017 Aug 23. J Clin Microbiol. 2017. PMID: 28835479 Free PMC article.
-
Imported arboviral infections in Italy, July 2014-October 2015: a National Reference Laboratory report.BMC Infect Dis. 2017 Mar 16;17(1):216. doi: 10.1186/s12879-017-2320-1. BMC Infect Dis. 2017. PMID: 28302072 Free PMC article.
-
External Quality Assessment (EQA) for Molecular Diagnostics of Zika Virus: Experiences from an International EQA Programme, 2016⁻2018.Viruses. 2018 Sep 13;10(9):491. doi: 10.3390/v10090491. Viruses. 2018. PMID: 30216988 Free PMC article. Review.
-
Preparedness of public health-care system for Zika virus outbreak: An Indian perspective.J Infect Public Health. 2020 Jul;13(7):949-955. doi: 10.1016/j.jiph.2020.03.016. Epub 2020 Apr 25. J Infect Public Health. 2020. PMID: 32340832 Review.
Cited by
-
Belgian rare diseases plan in clinical pathology: identification of key biochemical diagnostic tests and establishment of reference laboratories and financing conditions.Orphanet J Rare Dis. 2021 Feb 17;16(1):89. doi: 10.1186/s13023-021-01728-1. Orphanet J Rare Dis. 2021. PMID: 33596965 Free PMC article.
-
Study protocol for the multicentre cohorts of Zika virus infection in pregnant women, infants, and acute clinical cases in Latin America and the Caribbean: the ZIKAlliance consortium.BMC Infect Dis. 2019 Dec 26;19(1):1081. doi: 10.1186/s12879-019-4685-9. BMC Infect Dis. 2019. PMID: 31878895 Free PMC article.
-
Zika virus threshold determines transmission by European Aedes albopictus mosquitoes.Emerg Microbes Infect. 2019;8(1):1668-1678. doi: 10.1080/22221751.2019.1689797. Emerg Microbes Infect. 2019. PMID: 31735122 Free PMC article.
-
Low capacity for molecular detection of Alphaviruses other than Chikungunya virus in 23 European laboratories, March 2022.PLoS One. 2025 Feb 27;20(2):e0318602. doi: 10.1371/journal.pone.0318602. eCollection 2025. PLoS One. 2025. PMID: 40014625 Free PMC article.
-
Laboratory readiness and response for novel coronavirus (2019-nCoV) in expert laboratories in 30 EU/EEA countries, January 2020.Euro Surveill. 2020 Feb;25(6):2000082. doi: 10.2807/1560-7917.ES.2020.25.6.2000082. Epub 2020 Feb 11. Euro Surveill. 2020. PMID: 32046815 Free PMC article.
References
-
- World Health Organization (WHO). WHO statement on the first meeting of the International Health Regulations (2005) (IHR 2005) Emergency Committee on Zika virus and observed increase in neurological disorders and neonatal malformations. Geneva: WHO; 2016. Available from: http://www.who.int/mediacentre/news/statements/2016/1st-emergency-commit...
-
- Pan American Health Organization (PAHO). 2016: The year Zika evolved from an emergency into a long-term public health challenge. Washington: PAHO; 2016. Available from: http://www.paho.org/hq/index.php?option=com_content&view=article&id=1286...
-
- World Health Organization (WHO). Fifth meeting of the Emergency Commitee under the International Health Regulations (2005) regarding microcephaly, other neurological disorders and Zika virus. Geneva: WHO; 2016. Available from: http://www.who.int/mediacentre/news/statements/2016/zika-fifth-ec/en/
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

