Stilbene Boronic Acids Form a Covalent Bond with Human Transthyretin and Inhibit Its Aggregation
- PMID: 28920684
- PMCID: PMC5659721
- DOI: 10.1021/acs.jmedchem.7b00952
Stilbene Boronic Acids Form a Covalent Bond with Human Transthyretin and Inhibit Its Aggregation
Abstract
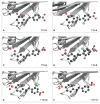
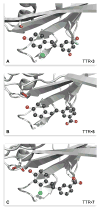
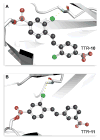
Transthyretin (TTR) is a homotetrameric protein. Its dissociation into monomers leads to the formation of fibrils that underlie human amyloidogenic diseases. The binding of small molecules to the thyroxin-binding sites in TTR stabilizes the homotetramer and attenuates TTR amyloidosis. Herein, we report on boronic acid-substituted stilbenes that limit TTR amyloidosis in vitro. Assays of affinity for TTR and inhibition of its tendency to form fibrils were coupled with X-ray crystallographic analysis of nine TTR·ligand complexes. The ensuing structure-function data led to a symmetrical diboronic acid that forms a boronic ester reversibly with serine 117. This diboronic acid inhibits fibril formation by both wild-type TTR and a common disease-related variant, V30M TTR, as effectively as does tafamidis, a small-molecule drug used to treat TTR-related amyloidosis in the clinic. These findings establish a new modality for covalent inhibition of fibril formation and illuminate a path for future optimization.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
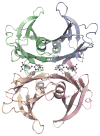





Similar articles
-
Monoaryl derivatives as transthyretin fibril formation inhibitors: Design, synthesis, biological evaluation and structural analysis.Bioorg Med Chem. 2020 Sep 15;28(18):115673. doi: 10.1016/j.bmc.2020.115673. Epub 2020 Jul 28. Bioorg Med Chem. 2020. PMID: 32828431
-
Conformational differences between the wild type and V30M mutant transthyretin modulate its binding to genistein: implications to tetramer stability and ligand-binding.J Struct Biol. 2010 Jun;170(3):522-31. doi: 10.1016/j.jsb.2010.03.002. Epub 2010 Mar 6. J Struct Biol. 2010. PMID: 20211733
-
Age-related oxidative modifications of transthyretin modulate its amyloidogenicity.Biochemistry. 2013 Mar 19;52(11):1913-26. doi: 10.1021/bi301313b. Epub 2013 Mar 4. Biochemistry. 2013. PMID: 23414091 Free PMC article.
-
Nearly 200 X-ray crystal structures of transthyretin: what do they tell us about this protein and the design of drugs for TTR amyloidoses?Curr Med Chem. 2012;19(15):2324-42. doi: 10.2174/092986712800269335. Curr Med Chem. 2012. PMID: 22471981 Review.
-
Methods to evaluate the inhibition of TTR fibrillogenesis induced by small ligands.Curr Med Chem. 2012;19(15):2343-55. doi: 10.2174/092986712800269281. Curr Med Chem. 2012. PMID: 22471983 Review.
Cited by
-
Boronic acid with high oxidative stability and utility in biological contexts.Proc Natl Acad Sci U S A. 2021 Mar 9;118(10):e2013691118. doi: 10.1073/pnas.2013691118. Proc Natl Acad Sci U S A. 2021. PMID: 33653951 Free PMC article.
-
Rational Design, Synthesis, Characterization and Evaluation of Iodinated 4,4'-Bipyridines as New Transthyretin Fibrillogenesis Inhibitors.Molecules. 2020 May 8;25(9):2213. doi: 10.3390/molecules25092213. Molecules. 2020. PMID: 32397334 Free PMC article.
-
Stimuli-Responsive Boron-Based Materials in Drug Delivery.Int J Mol Sci. 2023 Feb 1;24(3):2757. doi: 10.3390/ijms24032757. Int J Mol Sci. 2023. PMID: 36769081 Free PMC article. Review.
-
Potential of Boronic Acid Derivatization and Activity in Agrochemical Discovery.Molecules. 2025 Jul 18;30(14):3018. doi: 10.3390/molecules30143018. Molecules. 2025. PMID: 40733283 Free PMC article. Review.
-
Recent developments in the medicinal chemistry of single boron atom-containing compounds.Acta Pharm Sin B. 2021 Oct;11(10):3035-3059. doi: 10.1016/j.apsb.2021.01.010. Epub 2021 Jan 20. Acta Pharm Sin B. 2021. PMID: 34729302 Free PMC article. Review.
References
-
- Dobson CM. Protein folding and misfolding. Science. 2003;426:884–890. - PubMed
-
- Chiti F, Dobson CM. Protein misfolding, functional amyloid, and human disease. Annu Rev Biochem. 2006;75:333–366. - PubMed
-
- Knowles TP, Vendruscolo M, Dobson CM. The amyloid state and its association with protein misfolding diseases. Nat Rev Mol Cell Biol. 2014;15:384–396. - PubMed
-
- Richardson SJ. Cell and molecular biology of transthyretin and thyroid hormones. Int Rev Cytol. 2007;258:137–193. - PubMed
-
- Klabunde T, Petrassi HM, Oza VB, Raman P, Kelly JW, Sacchettini JC. Rational design of potent human transthyretin amyloid disease inhibitors. Nat Struct Biol. 2000;7:312–321. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Research Materials
Miscellaneous

