Impaired angiopoietin/Tie2 signaling compromises Schlemm's canal integrity and induces glaucoma
- PMID: 28920924
- PMCID: PMC5617682
- DOI: 10.1172/JCI94668
Impaired angiopoietin/Tie2 signaling compromises Schlemm's canal integrity and induces glaucoma
Abstract
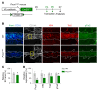
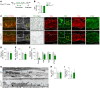
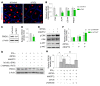
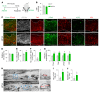
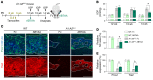
Primary open-angle glaucoma (POAG) is often caused by elevated intraocular pressure (IOP), which arises due to increased resistance to aqueous humor outflow (AHO). Aqueous humor flows through Schlemm's canal (SC), a lymphatic-like vessel encircling the cornea, and via intercellular spaces of ciliary muscle cells. However, the mechanisms underlying increased AHO resistance are poorly understood. Here, we demonstrate that signaling between angiopoietin (Angpt) and the Angpt receptor Tie2, which is critical for SC formation, is also indispensable for maintaining SC integrity during adulthood. Deletion of Angpt1/Angpt2 or Tie2 in adult mice severely impaired SC integrity and transcytosis, leading to elevated IOP, retinal neuron damage, and impairment of retinal ganglion cell function, all hallmarks of POAG in humans. We found that SC integrity is maintained by interconnected and coordinated functions of Angpt-Tie2 signaling, AHO, and Prox1 activity. These functions diminish in the SC during aging, leading to impaired integrity and transcytosis. Intriguingly, Tie2 reactivation using a Tie2 agonistic antibody rescued the POAG phenotype in Angpt1/Angpt2-deficient mice and rejuvenated the SC in aged mice. These results indicate that the Angpt-Tie2 system is essential for SC integrity. The impairment of this system underlies POAG-associated pathogenesis, supporting the possibility that Tie2 agonists could be a therapeutic option for glaucoma.
Conflict of interest statement
Figures







Comment in
- All TIEd up: mechanisms of Schlemm’s canal maintenance doi: 10.1172/JCI96840
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

