Lifelong haematopoiesis is established by hundreds of precursors throughout mammalian ontogeny
- PMID: 28920953
- PMCID: PMC5705075
- DOI: 10.1038/ncb3607
Lifelong haematopoiesis is established by hundreds of precursors throughout mammalian ontogeny
Abstract
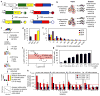
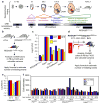
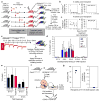
Current dogma asserts that mammalian lifelong blood production is established by a small number of blood progenitors. However, this model is based on assays that require the disruption, transplantation and/or culture of embryonic tissues. Here, we used the sample-to-sample variance of a multicoloured lineage trace reporter to assess the frequency of emerging lifelong blood progenitors while avoiding the disruption, culture or transplantation of embryos. We find that approximately 719 Flk1+ mesodermal precursors, 633 VE-cadherin+ endothelial precursors and 545 Vav1+ nascent blood stem and progenitor cells emerge to establish the haematopoietic system at embryonic days (E)7-E8.5, E8.5-E11.5 and E11.5-E14.5, respectively. We also determined that the spatio-temporal recruitment of endothelial blood precursors begins at E8.5 and ends by E10.5, and that many c-Kit+ clusters of newly specified blood progenitors in the aorta are polyclonal in origin. Our work illuminates the dynamics of the developing mammalian blood system during homeostasis.
Figures



References
-
- Medvinsky A, Rybtsov S, Taoudi S. Embryonic origin of the adult hematopoietic system: advances and questions. Development. 2011;138:1017–1031. doi:138/6/1017[pii]10.1242/dev.040998. - PubMed
-
- Kumaravelu P, et al. Quantitative developmental anatomy of definitive haematopoietic stem cells/long-term repopulating units (HSC/RUs): role of the aorta-gonad-mesonephros (AGM) region and the yolk sac in colonisation of the mouse embryonic liver. Development. 2002;129:4891–4899. - PubMed
-
- Medvinsky A, Dzierzak E. Definitive hematopoiesis is autonomously initiated by the AGM region. Cell. 1996;86:897–906. doi:S0092-8674(00)80165-8 [pii] - PubMed
-
- Muller AM, Medvinsky A, Strouboulis J, Grosveld F, Dzierzak E. Development of hematopoietic stem cell activity in the mouse embryo. Immunity. 1994;1:291–301. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

