Dendritic spines provide cognitive resilience against Alzheimer's disease
- PMID: 28921611
- PMCID: PMC5744899
- DOI: 10.1002/ana.25049
Dendritic spines provide cognitive resilience against Alzheimer's disease
Abstract
Objective: Neuroimaging and other biomarker assays suggest that the pathological processes of Alzheimer's disease (AD) begin years prior to clinical dementia onset. However, some 30 to 50% of older individuals who harbor AD pathology do not become symptomatic in their lifetime. It is hypothesized that such individuals exhibit cognitive resilience that protects against AD dementia. We hypothesized that in cases with AD pathology, structural changes in dendritic spines would distinguish individuals who had or did not have clinical dementia.
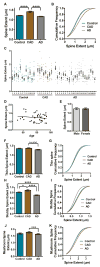
Methods: We compared dendritic spines within layer II and III pyramidal neuron dendrites in Brodmann area 46 dorsolateral prefrontal cortex using the Golgi-Cox technique in 12 age-matched pathology-free controls, 8 controls with AD pathology (CAD), and 21 AD cases. We used highly optimized methods to trace impregnated dendrites from bright-field microscopy images that enabled accurate 3-dimensional digital reconstruction of dendritic structure for morphologic analyses.
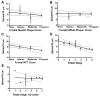
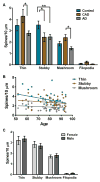
Results: Spine density was similar among control and CAD cases but was reduced significantly in AD. Thin and mushroom spines were reduced significantly in AD compared to CAD brains, whereas stubby spine density was decreased significantly in CAD and AD compared to controls. Increased spine extent distinguished CAD cases from controls and AD. Linear regression analysis of all cases indicated that spine density was not associated with neuritic plaque score but did display negative correlation with Braak staging.
Interpretation: These observations provide cellular evidence to support the hypothesis that dendritic spine plasticity is a mechanism of cognitive resilience that protects older individuals with AD pathology from developing dementia. Ann Neurol 2017;82:602-614.
© 2017 American Neurological Association.
Conflict of interest statement
Nothing to report.
Figures

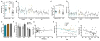

References
-
- Bennett DA, Schneider JA, Arvanitakis Z, et al. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology. 2006;66:1837–1844. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

