The role of the PI3K-Akt signaling pathway in the developmental competence of bovine oocytes
- PMID: 28922408
- PMCID: PMC5602670
- DOI: 10.1371/journal.pone.0185045
The role of the PI3K-Akt signaling pathway in the developmental competence of bovine oocytes
Abstract
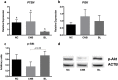
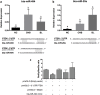
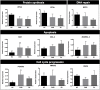
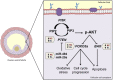
The ovarian follicle encloses oocytes in a microenvironment throughout their growth and acquisition of competence. Evidence suggests a dynamic interplay among follicular cells and oocytes, since they are constantly exchanging "messages". We dissected bovine ovarian follicles and recovered follicular cells (FCs-granulosa and cumulus cells) and cumulus-oocyte complexes (COCs) to investigate whether the PI3K-Akt signaling pathway impacted oocyte quality. Following follicle rupture, COCs were individually selected for in vitro cultures to track the follicular cells based on oocyte competence to reach the blastocyst stage after parthenogenetic activation. Levels of PI3K-Akt signaling pathway components in FCs correlated with oocyte competence. This pathway is upregulated in FCs from follicles with high-quality oocytes that are able to reach the blastocyst stage, as indicated by decreased levels of PTEN and increased levels of the PTEN regulators bta-miR-494 and bta-miR-20a. Using PI3K-Akt responsive genes, we showed decreased FOXO3a levels and BAX levels in lower quality groups, indicating changes in cell cycle progression, oxidative response and apoptosis. Based on these results, the measurement of levels of PI3K-Akt pathway components in FCs from ovarian follicles carrying oocytes with distinct developmental competences is a useful tool to identify putative molecular pathways involved in the acquisition of oocyte competence.
Conflict of interest statement
Figures
References
-
- Lonergan P, Rizos D, Gutierrez-Adan A, Fair T, Boland MP. Oocyte and embryo quality: Effect of origin, culture conditions and gene expression patterns. Reproduction in Domestic Animals. 2003;38(4):259–67. doi: 10.1046/j.1439-0531.2003.00437.x - DOI - PubMed
-
- Sirard MA, Dufort I, Coenen K, Tremblay K, Massicotte L, Robert C. The use of genomics and proteomics to understand oocyte and early embryo functions in farm animals. Reproduction. 2003:117–29. - PubMed
-
- Mermillod P, Dalbies-Tran R, Uzbekova S, Thelie A, Traverso JM, Perreau C, et al. Factors affecting oocyte quality: Who is driving the follicle? Reproduction in Domestic Animals. 2008;43:393–400. doi: 10.1111/j.1439-0531.2008.01190.x - DOI - PubMed
-
- Krisher RL. In Vivo and In Vitro Environmental Effects on Mammalian Oocyte Quality. Annual Review of Animal Biosciences, Vol 1. 2013;1:393–417. doi: 10.1146/annurev-animal-031412-103647 - DOI - PubMed
-
- Combelles CMH, Holick EA, Paolella LJ, Walker DC, Wu QQ. Profiling of superoxide dismutase isoenzymes in compartments of the developing bovine antral follicles. Reproduction. 2010;139(5):871–81. doi: 10.1530/REP-09-0390 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

