Direct-acting antivirals for chronic hepatitis C
- PMID: 28922704
- PMCID: PMC6484376
- DOI: 10.1002/14651858.CD012143.pub3
Direct-acting antivirals for chronic hepatitis C
Abstract
Background: Millions of people worldwide suffer from hepatitis C, which can lead to severe liver disease, liver cancer, and death. Direct-acting antivirals (DAAs), e.g. sofosbuvir, are relatively new and expensive interventions for chronic hepatitis C, and preliminary results suggest that DAAs may eradicate hepatitis C virus (HCV) from the blood (sustained virological response). Sustained virological response (SVR) is used by investigators and regulatory agencies as a surrogate outcome for morbidity and mortality, based solely on observational evidence. However, there have been no randomised trials that have validated that usage.
Objectives: To assess the benefits and harms of DAAs in people with chronic HCV.
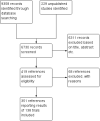
Search methods: We searched for all published and unpublished trials in The Cochrane Hepato-Biliary Group Controlled Trials Register, CENTRAL, MEDLINE, Embase, Science Citation Index Expanded, LILACS, and BIOSIS; the Chinese Biomedical Literature Database (CBM), China Network Knowledge Information (CNKI), the Chinese Science Journal Database (VIP), Google Scholar, The Turning Research into Practice (TRIP) Database, ClinicalTrials.gov, European Medicines Agency (EMA) (www.ema.europa.eu/ema/), WHO International Clinical Trials Registry Platform (www.who.int/ictrp), the Food and Drug Administration (FDA) (www.fda.gov), and pharmaceutical company sources for ongoing or unpublished trials. Searches were last run in October 2016.
Selection criteria: Randomised clinical trials comparing DAAs versus no intervention or placebo, alone or with co-interventions, in adults with chronic HCV. We included trials irrespective of publication type, publication status, and language.
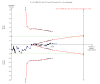
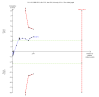
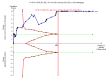
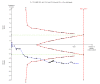
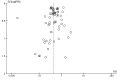
Data collection and analysis: We used standard methodological procedures expected by Cochrane. Our primary outcomes were hepatitis C-related morbidity, serious adverse events, and health-related quality of life. Our secondary outcomes were all-cause mortality, ascites, variceal bleeding, hepato-renal syndrome, hepatic encephalopathy, hepatocellular carcinoma, non-serious adverse events (each reported separately), and SVR. We systematically assessed risks of bias, performed Trial Sequential Analysis, and followed an eight-step procedure to assess thresholds for statistical and clinical significance. We evaluated the overall quality of the evidence, using GRADE.
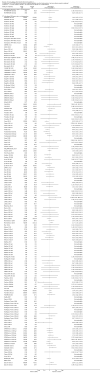
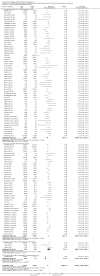
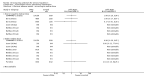
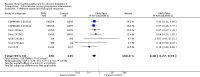
Main results: We included a total of 138 trials randomising a total of 25,232 participants. The trials were generally short-term trials and designed primarily to assess the effect of treatment on SVR. The trials evaluated 51 different DAAs. Of these, 128 trials employed matching placebo in the control group. All included trials were at high risk of bias. Eighty-four trials involved DAAs on the market or under development (13,466 participants). Fifty-seven trials administered DAAs that were discontinued or withdrawn from the market. Study populations were treatment-naive in 95 trials, had been exposed to treatment in 17 trials, and comprised both treatment-naive and treatment-experienced individuals in 24 trials. The HCV genotypes were genotype 1 (119 trials), genotype 2 (eight trials), genotype 3 (six trials), genotype 4 (nine trials), and genotype 6 (one trial). We identified two ongoing trials.We could not reliably determine the effect of DAAs on the market or under development on our primary outcome of hepatitis C-related morbidity or all-cause mortality. There were no data on hepatitis C-related morbidity and only limited data on mortality from 11 trials (DAA 15/2377 (0.63%) versus control 1/617 (0.16%); OR 3.72, 95% CI 0.53 to 26.18, very low-quality evidence). We did not perform Trial Sequential Analysis on this outcome.There is very low quality evidence that DAAs on the market or under development do not influence serious adverse events (DAA 5.2% versus control 5.6%; OR 0.93, 95% CI 0.75 to 1.15 , 15,817 participants, 43 trials). The Trial Sequential Analysis showed that there was sufficient information to rule out that DAAs reduce the relative risk of a serious adverse event by 20% when compared with placebo. The only DAA that showed a lower risk of serious adverse events when meta-analysed separately was simeprevir (OR 0.62, 95% CI 0.45 to 0.86). However, Trial Sequential Analysis showed that there was not enough information to confirm or reject a relative risk reduction of 20%, and when one trial with an extreme result was excluded, the meta-analysis result showed no evidence of a difference.DAAs on the market or under development may reduce the risk of no SVR from 54.1% in untreated people to 23.8% in people treated with DAA (RR 0.44, 95% CI 0.37 to 0.52, 6886 participants, 32 trials, low quality evidence). Trial Sequential Analysis confirmed this meta-analysis result.Only 1/84 trials on the market or under development assessed the effects of DAAs on health-related quality of life (SF-36 mental score and SF-36 physical score).There was insufficient evidence from trials on withdrawn or discontinued DAAs to determine their effect on hepatitis C-related morbidity and all-cause mortality (OR 0.64, 95% CI 0.23 to 1.79; 5 trials, very low-quality evidence). However, these DAAs seemed to increase the risk of serious adverse events (OR 1.45, 95% CI 1.22 to 1.73; 29 trials, very low-quality evidence). Trial Sequential Analysis confirmed this meta-analysis result.None of the 138 trials provided useful data to assess the effects of DAAs on the remaining secondary outcomes (ascites, variceal bleeding, hepato-renal syndrome, hepatic encephalopathy, and hepatocellular carcinoma).
Authors' conclusions: The evidence for our main outcomes of interest come from short-term trials, and we are unable to determine the effect of long-term treatment with DAAs. The rates of hepatitis C morbidity and mortality observed in the trials are relatively low and we are uncertain as to how DAAs affect this outcome. Overall, there is very low quality evidence that DAAs on the market or under development do not influence serious adverse events. There is insufficient evidence to judge if DAAs have beneficial or harmful effects on other clinical outcomes for chronic HCV. Simeprevir may have beneficial effects on risk of serious adverse event. In all remaining analyses, we could neither confirm nor reject that DAAs had any clinical effects. DAAs may reduce the number of people with detectable virus in their blood, but we do not have sufficient evidence from randomised trials that enables us to understand how SVR affects long-term clinical outcomes. SVR is still an outcome that needs proper validation in randomised clinical trials.
Conflict of interest statement
JCJ: none declared. EN: none declared. JF: none declared. KK: none declared. KF: none declared. GH: none declared. GP: none declared. SD: none declared. KW: none declared. MB: none declared. GB: none declared. SK: none declared. JP: none declared. DN: none declared. RK: none declared. CG: none declared.
Figures









































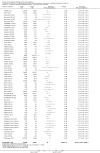
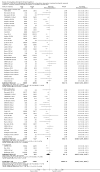
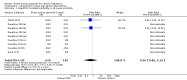

















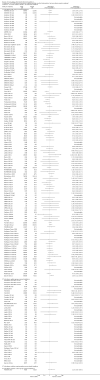























Update of
-
Direct-acting antivirals for chronic hepatitis C.Cochrane Database Syst Rev. 2017 Jun 6;6(6):CD012143. doi: 10.1002/14651858.CD012143.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2017 Sep 18;9:CD012143. doi: 10.1002/14651858.CD012143.pub3. PMID: 28585310 Free PMC article. Updated.
Comment in
-
The Cochrane Review Conclusion for Hepatitis C DAA Therapies is Wrong.Am J Gastroenterol. 2018 Jan;113(1):2-4. doi: 10.1038/ajg.2017.420. Epub 2017 Nov 14. Am J Gastroenterol. 2018. PMID: 29134963 No abstract available.
-
Who is wrong? Responses to Kwo et al.Am J Gastroenterol. 2018 May;113(5):779-780. doi: 10.1038/s41395-018-0029-4. Epub 2018 Feb 27. Am J Gastroenterol. 2018. PMID: 29487409 No abstract available.
-
Letter to Editor: Response to AASLD Editorial/Message from the President.Hepatology. 2019 May;69(5):2300. doi: 10.1002/hep.30298. Hepatology. 2019. PMID: 30276829
-
Reply.Hepatology. 2019 May;69(5):2301. doi: 10.1002/hep.30564. Hepatology. 2019. PMID: 30762891
References
References to studies included in this review
ADVANCE 2011a1 {published data only}
-
- Curtis S, Cure S, Gavart S, Dearden L, Fleischmann J, Ouwens M, et al. The cost‐effectiveness of telaprevir (TVR) in combination with pegylated interferon‐alfa and ribavirin (PR) for the treatment of genotype 1 chronic hepatitis C patients. Journal of Hepatology 2012; Vol. 56, issue Suppl 2:S434.
-
- Bisceglie AM, Dusheiko GM, Muir AJ, Zeuzem S, Marcellin P, Bzowej NH. Telaprevir in combination with peginterferon alfa‐2a and ribavirin: analyses of pre‐defined subpopulations in the phase 3 ADVANCE trial. Gastroenterology 2011;140(5 Suppl 1):S908.
-
- Dusheiko GM, Bengtsson L, Adda N, Kauffman R, Jacobson IM. Telaprevir in combination with peginterferon and ribavirin in genotype 1 HCV treatment‐naive patients: final results of phase 3 Advance study. Gut 2011;60(Suppl 2):A32‐3.
-
- Everson G, Dusheiko G, Pol S, Nelson D, Muir A, Andreone P, et al. Telaprevir in combination with peginterferon alfa‐2a and ribavirin increased sustained virologic response in genotype 1 chronic HCV and allowed for shorter treatment duration in treatment‐naive and prior relapser patients. American Journal of Gastroenterology 2011;106(2S):S113‐4.
ADVANCE 2011a2 {published data only}
-
- Curtis S, Cure S, Gavart S, Dearden L, Fleischmann J, Ouwens M, et al. The cost‐effectiveness of telaprevir (TVR) in combination with pegylated interferon‐alfa and ribavirin (PR) for the treatment of genotype 1 chronic hepatitis C patients. Journal of Hepatology 2012; Vol. 56, issue Suppl 2:S434.
-
- Bisceglie AM, Dusheiko GM, Muir AJ, Zeuzem S, Marcellin P, Bzowej NH. Telaprevir in combination with peginterferon alfa‐2a and ribavirin: analyses of pre‐defined subpopulations in the phase 3 ADVANCE trial. Gastroenterology 2011;140(5 Suppl 1):S908.
-
- Dusheiko GM, Bengtsson L, Adda N, Kauffman R, Jacobson IM. Telaprevir in combination with peginterferon and ribavirin in genotype 1 HCV treatment‐naive patients: final results of phase 3 Advance study. Gut 2011;60(Suppl 2):A32‐3.
-
- Everson G, Dusheiko G, Pol S, Nelson D, Muir A, Andreone P, et al. Telaprevir in combination with peginterferon alfa‐2a and ribavirin increased sustained virologic response in genotype 1 chronic HCV and allowed for shorter treatment duration in treatment‐naive and prior relapser patients. American Journal of Gastroenterology 2011;106(2S):S113‐4.
Anderson 2014a1 {published data only}
-
- Anderson RT, Baran RW, Erickson P, Revicki DA, Dietz B, Gooch K. Psychometric evaluation of the hepatitis C virus patient‐reported outcomes (HCV‐PRO) instrument: validity, responsiveness, and identification of the minimally important difference in a phase 2 clinical trial. Quality of Life Research 2014;23(3):877‐86. - PubMed
-
- Gaultier I, Cohen DE, Bhathena A, Idler K, Larsen LM, Podsadecki T, et al. The effect of IL28B polymorphisms on virologic response to treatment with pegylated interferon alpha‐2A and ribavirin (SOC) added to ABT‐450/ritonavir (ABT‐450/R), ABT‐333, or ABT‐072. Journal of Hepatology 2011;54(Suppl 1):S523.
-
- Pilot‐Matias TJ, Tripathi RL, Dekhtyar T, Menon RM, Gaultier IA, Cohen DE, et al. Genotypic and phenotypic characterization of NS3 variants selected in HCV‐infected patients treated with ABT‐450. Journal of Hepatology 2011;54(Suppl 1):S485‐6.
Anderson 2014a2 {published data only}
-
- Anderson RT, Baran RW, Erickson P, Revicki DA, Dietz B, Gooch K. Psychometric evaluation of the hepatitis C virus patient‐reported outcomes (HCV‐PRO) instrument: validity, responsiveness, and identification of the minimally important difference in a phase 2 clinical trial. Quality of Life Research 2014;23(3):877‐86. - PubMed
-
- Gaultier I, Cohen DE, Bhathena A, Idler K, Larsen LM, Podsadecki T, et al. The effect of IL28B polymorphisms on virologic response to treatment with pegylated interferon alpha‐2A and ribavirin (SOC) added to ABT‐450/ritonavir (ABT‐450/R), ABT‐333, or ABT‐072. Journal of Hepatology 2011;54(Suppl 1):S523.
-
- Pilot‐Matias TJ, Tripathi RL, Dekhtyar T, Menon RM, Gaultier IA, Cohen DE, et al. Genotypic and phenotypic characterization of NS3 variants selected in HCV‐infected patients treated with ABT‐450. Journal of Hepatology 2011;54(Suppl 1):S485‐6.
Anderson 2014a3 {published data only}
-
- Anderson RT, Baran RW, Erickson P, Revicki DA, Dietz B, Gooch K. Psychometric evaluation of the hepatitis C virus patient‐reported outcomes (HCV‐PRO) instrument: validity, responsiveness, and identification of the minimally important difference in a phase 2 clinical trial. Quality of Life Research 2014;23(3):877‐86. - PubMed
-
- Gaultier I, Cohen DE, Bhathena A, Idler K, Larsen LM, Podsadecki T, et al. The effect of IL28B polymorphisms on virologic response to treatment with pegylated interferon alpha‐2A and ribavirin (SOC) added to ABT‐450/ritonavir (ABT‐450/R), ABT‐333, or ABT‐072. Journal of Hepatology 2011;54(Suppl 1):S523.
-
- Pilot‐Matias TJ, Tripathi RL, Dekhtyar T, Menon RM, Gaultier IA, Cohen DE, et al. Genotypic and phenotypic characterization of NS3 variants selected in HCV‐infected patients treated with ABT‐450. Journal of Hepatology 2011;54(Suppl 1):S485‐6.
Anderson 2014a4 {published data only}
-
- Anderson RT, Baran RW, Erickson P, Revicki DA, Dietz B, Gooch K. Psychometric evaluation of the hepatitis C virus patient‐reported outcomes (HCV‐PRO) instrument: validity, responsiveness, and identification of the minimally important difference in a phase 2 clinical trial. Quality of Life Research 2014;23(3):877‐86. - PubMed
-
- Gaultier I, Cohen DE, Bhathena A, Idler K, Larsen LM, Podsadecki T, et al. The effect of IL28B polymorphisms on virologic response to treatment with pegylated interferon alpha‐2A and ribavirin (SOC) added to ABT‐450/ritonavir (ABT‐450/R), ABT‐333, or ABT‐072. Journal of Hepatology 2011;54(Suppl 1):S523.
-
- Pilot‐Matias TJ, Tripathi RL, Dekhtyar T, Menon RM, Gaultier IA, Cohen DE, et al. Genotypic and phenotypic characterization of NS3 variants selected in HCV‐infected patients treated with ABT‐450. Journal of Hepatology 2011;54(Suppl 1):S485‐6.
Anderson 2014a5 {published data only}
-
- Anderson RT, Baran RW, Erickson P, Revicki DA, Dietz B, Gooch K. Psychometric evaluation of the hepatitis C virus patient‐reported outcomes (HCV‐PRO) instrument: validity, responsiveness, and identification of the minimally important difference in a phase 2 clinical trial. Quality of Life Research 2014;23(3):877‐86. - PubMed
-
- Gaultier I, Cohen DE, Bhathena A, Idler K, Larsen LM, Podsadecki T, et al. The effect of IL28B polymorphisms on virologic response to treatment with pegylated interferon alpha‐2A and ribavirin (SOC) added to ABT‐450/ritonavir (ABT‐450/R), ABT‐333, or ABT‐072. Journal of Hepatology 2011;54(Suppl 1):S523.
-
- Pilot‐Matias TJ, Tripathi RL, Dekhtyar T, Menon RM, Gaultier IA, Cohen DE, et al. Genotypic and phenotypic characterization of NS3 variants selected in HCV‐infected patients treated with ABT‐450. Journal of Hepatology 2011;54(Suppl 1):S485‐6.
Anderson 2014a6 {published data only}
-
- Anderson RT, Baran RW, Erickson P, Revicki DA, Dietz B, Gooch K. Psychometric evaluation of the hepatitis C virus patient‐reported outcomes (HCV‐PRO) instrument: validity, responsiveness, and identification of the minimally important difference in a phase 2 clinical trial. Quality of Life Research 2014;23(3):877‐86. - PubMed
-
- Gaultier I, Cohen DE, Bhathena A, Idler K, Larsen LM, Podsadecki T, et al. The effect of IL28B polymorphisms on virologic response to treatment with pegylated interferon alpha‐2A and ribavirin (SOC) added to ABT‐450/ritonavir (ABT‐450/R), ABT‐333, or ABT‐072. Journal of Hepatology 2011;54(Suppl 1):S523.
-
- Pilot‐Matias TJ, Tripathi RL, Dekhtyar T, Menon RM, Gaultier IA, Cohen DE, et al. Genotypic and phenotypic characterization of NS3 variants selected in HCV‐infected patients treated with ABT‐450. Journal of Hepatology 2011;54(Suppl 1):S485‐6.
Anderson 2014a7 {published data only}
-
- Anderson RT, Baran RW, Erickson P, Revicki DA, Dietz B, Gooch K. Psychometric evaluation of the hepatitis C virus patient‐reported outcomes (HCV‐PRO) instrument: validity, responsiveness, and identification of the minimally important difference in a phase 2 clinical trial. Quality of Life Research 2014;23(3):877‐86. - PubMed
-
- Gaultier I, Cohen DE, Bhathena A, Idler K, Larsen LM, Podsadecki T, et al. The effect of IL28B polymorphisms on virologic response to treatment with pegylated interferon alpha‐2A and ribavirin (SOC) added to ABT‐450/ritonavir (ABT‐450/R), ABT‐333, or ABT‐072. Journal of Hepatology 2011;54(Suppl 1):S523.
-
- Pilot‐Matias TJ, Tripathi RL, Dekhtyar T, Menon RM, Gaultier IA, Cohen DE, et al. Genotypic and phenotypic characterization of NS3 variants selected in HCV‐infected patients treated with ABT‐450. Journal of Hepatology 2011;54(Suppl 1):S485‐6.
Anderson 2014a8 {published data only}
-
- Anderson RT, Baran RW, Erickson P, Revicki DA, Dietz B, Gooch K. Psychometric evaluation of the hepatitis C virus patient‐reported outcomes (HCV‐PRO) instrument: validity, responsiveness, and identification of the minimally important difference in a phase 2 clinical trial. Quality of Life Research 2014;23(3):877‐86. - PubMed
-
- Gaultier I, Cohen DE, Bhathena A, Idler K, Larsen LM, Podsadecki T, et al. The effect of IL28B polymorphisms on virologic response to treatment with pegylated interferon alpha‐2A and ribavirin (SOC) added to ABT‐450/ritonavir (ABT‐450/R), ABT‐333, or ABT‐072. Journal of Hepatology 2011;54(Suppl 1):S523.
-
- Pilot‐Matias TJ, Tripathi RL, Dekhtyar T, Menon RM, Gaultier IA, Cohen DE, et al. Genotypic and phenotypic characterization of NS3 variants selected in HCV‐infected patients treated with ABT‐450. Journal of Hepatology 2011;54(Suppl 1):S485‐6.
Anonymous (PPI‐461) 2011a1 {unpublished data only}
-
- NCT01247194. A phase 1b study of PPI‐461 in patients with HCV genotype 1. clinicaltrials.gov/ct2/show/study/NCT01247194?term=NCT01247194&rank=1 (first received 22 November 2010).
Anonymous (PPI‐461) 2011a2 {unpublished data only}
-
- NCT01247194. A phase 1b study of PPI‐461 in patients with HCV genotype 1. clinicaltrials.gov/ct2/show/study/NCT01247194?term=NCT01247194&rank=1 (first received 22 November 2010).
Anonymous (PPI‐461) 2011a3 {unpublished data only}
-
- NCT01247194. A phase 1b study of PPI‐461 in patients with HCV genotype 1. clinicaltrials.gov/ct2/show/study/NCT01247194?term=NCT01247194&rank=1 (first received 22 November 2010).
ASPIRE 2014 {published data only}
-
- Ferenci P, Fried M, Poordad F, Zeuzem S, Lenz O, Ouwerkerk‐Mahadevan S, et al. Safety of simeprevir (SMV) with peg Ifnalpha‐2a/ribavirin (PR) in HCV genotype 1‐infected patients: PILLAR and ASPIRE trials. Swiss Medical Weekly 2013;143(Suppl 199):15S.
-
- Lenz O, Fevery B, Vijgen L, Verbeeck J, Peeters M, Beumont‐Mauviel M, et al. TMC435 in patients infected with HCV genotype 1 who have failed previous pegylated interferon/ribavirin treatment: virologic analyses of the ASPIRE trial. Journal of Hepatology 2012;56(Suppl S2):S5.
-
- Scott J, Rosa K, Fu M, Cerri K, Peeters M, Beumont M, et al. Fatigue during treatment for hepatitis C virus: results of self‐reported fatigue severity in two Phase IIb studies of simeprevir treatment in patients with hepatitis C virus genotype 1 infection. BMC Infectious Diseases 2014;14:465. - PMC - PubMed
-
- Scott J, Rosa K, Fu M, Cerri K, Peeters M, Beumont‐Mauviel M, et al. Fatigue severity scale: reliability, validity and interpretation of change‐evidence from two clinical trials in patients with chronic HCV infection. Value in Health 2013;16(3):A216.
-
- Scott J, Rosa K, Zeuzem S, Beumont‐Mauviel M, Peeters M, Cerri, K, et al. Adding simeprevir (TMC435) to pegylated interferon/ribavirin does not increase patient reported fatigue in treatment‐experienced patients with chronic HCV infection: results from the ASPIRE trial. Journal of Hepatology 2013;58(Suppl S1):S371.
ATLAS 2013 {published data only}
-
- Marcellin P, Cooper C, Balart L, Larrey D, Box T, Yoshida E, et al. Randomized controlled trial of danoprevir plus peginterferon alfa‐2a and ribavirin in treatment‐naïve patients with hepatitis C virus genotype 1 infection. Gastroenterology 2013;145:790‐800. - PubMed
-
- Marcellin P, Cooper C, Balart LA, Larrey DG, Box TD, Yoshida E, et al. High sustained virological response rates with response‐guided danoprevir plus peginterferon alfa‐2a (40KD) and ribavirin in treatment‐naive, HCV genotype 1 patients: ATLAS study final results. Journal of Hepatology 2012;56(Suppl S2):S472.
-
- Terrault N, Cooper C, Balart LA, Larrey DG, Box TD, Yoshida E, et al. High sustained virologic response (SVR24) rates with response‐guided danoprevir (DNV; RG7227) plus pegIfn alpha‐2A (40KD) and ribavirin (P/R) in treatment‐naive HCV genotype 1 (G1) patients: results from the ATLAS study. Hepatology 2011;54(S1):398A‐9A.
-
- Terrault N, Cooper C, Balart LA, Larrey DG, Box TD, Yoshida EM, et al. Phase II randomised, partially‐blind, parallel‐group study of oral danoprevir (RG7227) with PegIFNα‐2a (Pegasys) plus ribavirin (Copegus) in treatment‐naive genotype 1 patients with CHC: results of planned week 12 interim analysis of the ATLAS study. Hepatology 2010;52(Suppl S1):335A.
Bacon 2011a1 {published data only}
-
- Bacon BR, Bruno S, Schiff ER, Kwo PY, Buti M, Pedicone L, et al. Predictors of sustained virologic response (SVR) among poor interferon (IFN) responders when boceprevir (BOC) is added to peginterferon alfa‐2b/ribavirin (PR). Hepatology 2011;54(S1):376A‐7A.
-
- Bacon BR, Gordon SC, Lawitz E, Marcellin P, Vierling JM, Zeuzem S, et al. HCV RESPOND‐2 final results: high sustained virologic response among genotype 1 previous non‐responders and relapsers to peginterferon/ribavirin when re‐treated with boceprevir plus PEGINTRON (peginterferon alfa‐2b)/ribavirin. Hepatology 2010;52(Suppl S1):430A.
-
- Barnard RJ, Howe JA, Ogert RA, Zeuzem S, Poordad F, Gordon SC, et al. Analysis of boceprevir resistance associated amino acid variants (RAVs) in two phase 3 boceprevir clinical studies. Virology 2013;444(1‐2):329‐36. - PubMed
-
- Bognar FA, Hu KQ, Thompson S, Pedicone L, Wahl J, Lin SG. Boceprevir (BOC) plus peginterferon alfa‐2b/ ribavirin (PR) in the treatment of chronic hepatitis C virus genotype‐1 (HCV‐G1) infected Asian patients in the SPRINT‐1, SPRINT‐2 and RESPOND‐2 trials. Journal of Gastroenterology and Hepatology 2012;27(Suppl 4):161.
Bacon 2011a2 {published data only}
-
- Bacon BR, Bruno S, Schiff ER, Kwo PY, Buti M, Pedicone L, et al. Predictors of sustained virologic response (SVR) among poor interferon (IFN) responders when boceprevir (BOC) is added to peginterferon alfa‐2b/ribavirin (PR). Hepatology 2011;54(S1):376A‐7A.
-
- Bacon BR, Gordon SC, Lawitz E, Marcellin P, Vierling JM, Zeuzem S, et al. HCV RESPOND‐2 final results: high sustained virologic response among genotype 1 previous non‐responders and relapsers to peginterferon/ribavirin when re‐treated with boceprevir plus PEGINTRON (peginterferon alfa‐2b)/ribavirin. Hepatology 2010;52(Suppl S1):430A.
-
- Barnard RJ, Howe JA, Ogert RA, Zeuzem S, Poordad F, Gordon SC, et al. Analysis of boceprevir resistance associated amino acid variants (RAVs) in two phase 3 boceprevir clinical studies. Virology 2013;444(1‐2):329‐36. - PubMed
-
- Bognar FA, Hu KQ, Thompson S, Pedicone L, Wahl J, Lin SG. Boceprevir (BOC) plus peginterferon alfa‐2b/ ribavirin (PR) in the treatment of chronic hepatitis C virus genotype‐1 (HCV‐G1) infected Asian patients in the SPRINT‐1, SPRINT‐2 and RESPOND‐2 trials. Journal of Gastroenterology and Hepatology 2012;27(Suppl 4):161.
Basu 2014a {published data only}
-
- Basu P, Shah N, Aloysius M. Simeprevir and sofosbuvir with modified doses of ribavirin (RBV) therapy on telaprevir‐experienced, coinfected (with HIV) cirrhotics with chronic hepatitis C (CHC): a randomized, open‐label, clinical pilot study, STOP C, interim results. American Journal of Gastroenterology 2014;109(Suppl. 2):S177.
-
- Basu P, Shah NJ, Aloysius MM, Brown RS Jr. Interferon ineligible naive chronic hepatitis C genotype I subjects treated with simeprevir and sofosbuvir in special population (psychiatric). A clinical pilot study; Inspire C study; interim results. HPB: the Official Journal of the International Hepato Pancreato Biliary Association 2015;17(Suppl S2):46.
Bavisotto 2007 {published data only}
-
- Bavisotto L, Wang CC, Jacobson IM, Marcellin P, Zeuzem S, Lawitz EJ, et al. Antiviral, pharmacokinetic and safety data for GS‐9190, a non‐nucleoside HCV NS5b polymerase inhibitor, in a phase‐1 trial in HCV genotype 1 infected subjects. Hepatology 2007;46(4 (Suppl 1)):255A.
-
- Harris J, Bae A, Sun SC, Svarovskaia ES, Miller MD, Mo H, et al. Antiviral response and resistance analysis of treatment‐naive HCV infected subjects receiving single and multiple doses of GS ‐ 9190. Hepatology 2010;52(Suppl S1):722A.
Benhamou 2013a1 {published data only}
-
- Benhamou Y, Moussalli J, Ratziu V, Lebray P, Backer K, Meyer S, et al. Telaprevir activity in treatment‐naive patients infected hepatitis C virus genotype 4: a randomized trial. Journal of Infectious Diseases 2013;208(6):1000‐7. - PubMed
-
- Benhamou Y, Moussalli J, Ratziu V, Lebray P, Gysen V, Backer K, et al. Results of a proof of concept study (C210) of telaprevir monotherapy and in combination with peginterferon ALFA‐2A and ribavirin in treatment‐naive genotype 4 HCV patients. Journal of Hepatology 2009;50(Suppl 1):S6.
-
- Benhamou Y, Moussalli J, Ratziu V, Lebray P, Backer K, Ghys A, et al. Activity of telaprevir monotherapy or in combination with peginterferon‐alfa‐2a and ribavirin in treatment‐naive genotype 4 hepatitis C patients: final results of study C210. Hepatology 2010;52(Suppl S1):719A.
Benhamou 2013a2 {published data only}
-
- Benhamou Y, Moussalli J, Ratziu V, Lebray P, Backer K, Meyer S, et al. Telaprevir activity in treatment‐naive patients infected hepatitis C virus genotype 4: a randomized trial. Journal of Infectious Diseases 2013;208(6):1000‐7. - PubMed
-
- Benhamou Y, Moussalli J, Ratziu V, Lebray P, Gysen V, Backer K, et al. Results of a proof of concept study (C210) of telaprevir monotherapy and in combination with peginterferon ALFA‐2A and ribavirin in treatment‐naive genotype 4 HCV patients. Journal of Hepatology 2009;50(Suppl 1):S6.
-
- Benhamou Y, Moussalli J, Ratziu V, Lebray P, Backer K, Ghys A, et al. Activity of telaprevir monotherapy or in combination with peginterferon‐alfa‐2a and ribavirin in treatment‐naive genotype 4 hepatitis C patients: final results of study C210. Hepatology 2010;52(Suppl S1):719A.
Boehringer Ingelheim 2010a {published data only}
-
- Boehringer Ingelheim. Safety, antiviral activity, and pharmcokinetics of multiple rising oral doses of BI201334 NA in treatment‐naive patients with chronic hepatitis C infection for 14 days monotherapy followed by combination with pegylated interferon and ribavirin for an additional 14 days (double‐blind, placebo controlled); and in treatment experienced patients with chronic hepatitis C infection for 28 days as combination therapy with pegylated interferon and ribavirin (open label). www.trials.boehringer‐ingelheim.com/public/trial_results_documents/1220/1220.2_U10‐2363‐01_PE_... (accessed 30 March 2016).
Boehringer Ingelheim 2010b {published data only}
-
- Boehringer Ingelheim. A randomised, double‐blind, placebo controlled trial with 200 mg BILN 2061ZW given p.o. at two consecutive days bid to investigate the antiviral efficacy, pharmacokinetics, safety in patients with cirrhosis and chronic hepatitis C. www.trials.boehringer‐ingelheim.com/public/trial_results_documents/605/605.9_U03‐1671.pdf (accessed prior to 27 March 2017.
Bronowicki 2013a1 {published data only}
-
- Bronowicki JP, Pol S, Thuluvath P, Larrey D, Martorell CT, Rustgi VK, et al. Asunaprevir (ASV; BMS‐650032), an NS3 protease inhibitor, in combination with peginterferon and ribavirin in treatment‐naive patients with genotype 1 chronic hepatitis C infection. Journal of Hepatology 2012;56(Suppl 2):S431‐2.
-
- Bronowicki JP, Pol S, Thuluvath PJ, Larrey D, Martorell CT, Rustgi VK, et al. BMS‐650032, an NS3 inhibitor, in combination with peginterferon alpha‐2a and ribavirin in treatment‐naive subjects with genotype 1 chronic hepatitis C infection. Journal of Hepatology 2011;54:S472.
-
- Bronowicki JP, Pol S, Thuluvath PJ, Larrey D, Martorell CT, Rustgi VK, et al. Randomized study of asunaprevir plus pegylated interferon‐alpha and ribavirin for previously untreated genotype 1 chronic hepatitis C. Antiviral Therapy 2013;18(7):885‐93. - PubMed
Bronowicki 2013a2 {published data only}
-
- Bronowicki JP, Pol S, Thuluvath P, Larrey D, Martorell CT, Rustgi VK, et al. Asunaprevir (ASV; BMS‐650032), an NS3 protease inhibitor, in combination with peginterferon and ribavirin in treatment‐naive patients with genotype 1 chronic hepatitis C infection. Journal of Hepatology 2012;56(Suppl 2):S431‐2.
-
- Bronowicki JP, Pol S, Thuluvath PJ, Larrey D, Martorell CT, Rustgi VK, et al. BMS‐650032, an NS3 inhibitor, in combination with peginterferon alpha‐2a and ribavirin in treatment‐naive subjects with genotype 1 chronic hepatitis C infection. Journal of Hepatology 2011;54:S472.
-
- Bronowicki JP, Pol S, Thuluvath PJ, Larrey D, Martorell CT, Rustgi VK, et al. Randomized study of asunaprevir plus pegylated interferon‐alpha and ribavirin for previously untreated genotype 1 chronic hepatitis C. Antiviral Therapy 2013;18(7):885‐93. - PubMed
Bronowicki 2013a3 {published data only}
-
- Bronowicki JP, Pol S, Thuluvath P, Larrey D, Martorell CT, Rustgi VK, et al. Asunaprevir (ASV; BMS‐650032), an NS3 protease inhibitor, in combination with peginterferon and ribavirin in treatment‐naive patients with genotype 1 chronic hepatitis C infection. Journal of Hepatology 2012;56(Suppl 2):S431‐2.
-
- Bronowicki JP, Pol S, Thuluvath PJ, Larrey D, Martorell CT, Rustgi VK, et al. BMS‐650032, an NS3 inhibitor, in combination with peginterferon alpha‐2a and ribavirin in treatment‐naive subjects with genotype 1 chronic hepatitis C infection. Journal of Hepatology 2011;54:S472.
-
- Bronowicki JP, Pol S, Thuluvath PJ, Larrey D, Martorell CT, Rustgi VK, et al. Randomized study of asunaprevir plus pegylated interferon‐alpha and ribavirin for previously untreated genotype 1 chronic hepatitis C. Antiviral Therapy 2013;18(7):885‐93. - PubMed
Bronowicki 2014 {published data only}
-
- Bronowicki J‐P, Ratziu V, Gadano A, Thuluvath Paul J, Bessone F, Martorell Claudia T, et al. Randomized trial of asunaprevir plus peginterferon alfa and ribavirin for previously untreated genotype 1 or 4 chronic hepatitis C. Journal of Hepatology 2014;61(6):1220‐7. - PubMed
-
- Bronowicki JP, Pol S, Thuluvath P, Larrey D, Martorell CT, Rustgi VK, et al. Asunaprevir (ASV; BMS‐650032), an NS3 protease inhibitor, in combination with peginterferon and ribavirin in treatment‐naive patients with genotype 1 chronic hepatitis C infection. Journal of Hepatology 2012;56(Suppl 2):S431‐S2.
-
- Bronowicki JP, Ratziu V, Gadano A, Thuluvath PJ, Bessone F, Martorell CT, et al. Asunaprevir with peginterferon‐alfa and ribavirin in treatment‐naive patients with genotype‐1 or ‐4 chronic hepatitis C: SVR24 results from a randomized phase 2B study (AI447016). Journal of Hepatology 2013;58:S571‐2.
C‐EDGE CO STAR 2015 {published data only}
-
- Dore G, Altice F, Litwin AH, Dalgard O, Gane EJ, Shibolet O, et al. C‐edge Co‐star: efficacy of grazoprevir and elbasvir in persons who inject drugs (PWID) receiving opioid agonist therapy. Hepatology 2015;62(Suppl S1):227A‐8A.
C‐EDGE TN 2015 {published data only}
-
- Arduino JM, Wang Y, Brown DD, Khawaja S, Martinez E, Butterton JR, et al. C‐EDGE TN: Impact of 12‐week oral regimen of grazoprevir (GZR, MK‐5172)/Elbasvir (EBR, MK‐8742) on patient‐reported outcomes (PROs) in treatment‐naive patients with chronic hepatitis C virus (HCV) genotype (GT) 1, 4, or 6 infection. Hepatology 2015;62(Suppl S1):565A‐566A.
-
- Zeuzem S, Ghalib R, Reddy K, Pockros PJ, Ben Ari ZIV, Zhao YUE, et al. Grazoprevir‐elbasvir combination therapy for treatment‐naive cirrhotic and noncirrhotic patients with chronic hepatitis C virus genotype 1, 4, or 6 infection: a randomized trial. Annals of Internal Medicine 2015;163(1):1‐13. - PubMed
-
- Zeuzem S, Ghalib R, Reddy KR, Pockros PJ, Ari ZB, Zhao Y, et al. The phase 3 C‐EDGE treatment‐naive (TN) study of a 12‐week oral regimen of grazoprevir (GZR, mk‐5172)/ elbasvir (EBR, mk‐8742) in patients with chronic HCV genotype (GT) 1, 4, or 6 infection. Journal of Hepatology 2015;62(Suppl S2):S213.
Chandra 2006a {published data only}
-
- Chandra P, Raible D, Harper D, Speth J, Villano S, Bichier G. Antiviral activity of the non‐nucleoside polymerase inhibitor, HCV‐796, in patients with chronic hepatitis C virus: preliminary results from a randomized, double‐blind, placebo‐controlled, ascending multiple dose study ‐ first article. Gastroenterology 2006;130(4 (Suppl 2)):A748.
COMMAND‐1 2015a1 {published data only}
-
- Hezode C, Hirschfield GM, Ghesquiere W, Sievert W, Rodriguez‐Torres M, Shafran S, et al. BMS‐790052, a NS5A replication complex inhibitor, combined with peginterferon alfa‐2a and ribavirin in treatment‐naive HCV‐genotype 1 or 4 patients: phase 2b AI444010 study interim week 12 results. Hepatology 2011;54(S1):474A‐5A.
-
- Hezode C, Hirschfield GM, Ghesquiere W, Sievert W, Rodriguez‐Torres M, Shafran S, et al. Daclatasvir (BMS‐790052), a NS5A replication complex inhibitor, combined with peginterferon‐alfa‐2a and ribavirin in treatment‐naive HCV genotype 1 or 4 subjects: phase 2b AI444010 study interim week 24 results. Hepatology International 2012;6(1):167.
-
- Hezode C, Hirschfield GM, Ghesquiere W, Sievert W, Rodriguez‐Torres M, Shafran SD, et al. Daclatasvir plus peginterferon alfa and ribavirin for treatment‐naive chronic hepatitis C genotype 1 or 4 infection: a randomised study. Gut 2015;64(6):948‐56. - PubMed
COMMAND‐1 2015a2 {published data only}
-
- Hezode C, Hirschfield GM, Ghesquiere W, Sievert W, Rodriguez‐Torres M, Shafran S, et al. BMS‐790052, a NS5A replication complex inhibitor, combined with peginterferon alfa‐2a and ribavirin in treatment‐naive HCV‐genotype 1 or 4 patients: phase 2b AI444010 study interim week 12 results. Hepatology 2011;54(S1):474A‐5A.
-
- Hezode C, Hirschfield GM, Ghesquiere W, Sievert W, Rodriguez‐Torres M, Shafran S, et al. Daclatasvir (BMS‐790052), a NS5A replication complex inhibitor, combined with peginterferon‐alfa‐2a and ribavirin in treatment‐naive HCV genotype 1 or 4 subjects: phase 2b AI444010 study interim week 24 results. Hepatology International 2012;6(1):167.
-
- Hezode C, Hirschfield GM, Ghesquiere W, Sievert W, Rodriguez‐Torres M, Shafran SD, et al. Daclatasvir plus peginterferon alfa and ribavirin for treatment‐naive chronic hepatitis C genotype 1 or 4 infection: a randomised study. Gut 2015;64(6):948‐56. - PubMed
CONCERTO‐1 2015 {published data only}
-
- Hayashi N, Izumi N, Kumada H, Okanoue T, Tsubouchi H, Yatsuhashi H, et al. Simeprevir with peginterferon/ribavirin for treatment‐naive hepatitis C genotype 1 patients in Japan: CONCERTO‐1, a phase III trial. Journal of Hepatology 2014;61(2):219‐27. - PubMed
Cooper 2009 {published data only}
-
- Cooper C, Lawitz EJ, Ghali P, Rodriguez‐Torres M, Anderson FH, Lee SS, et al. Antiviral activity of the non‐nucleoside polymerase inhibitor, VCH‐759, in chronic hepatitis C patients: results from a randomized, double‐blind, placebo‐controlled, ascending multiple dose study. Hepatology 2007;46(4 (Suppl 1)):864A.
-
- Cooper C, Lawitz EJ, Ghali P, Rodriguez‐Torres M, Anderson FH, Lee SS, et al. Evaluation of VCH‐759 monotherapy in hepatitis C infection. Journal of Hepatology 2009;51(1):39‐46. - PubMed
Dauphine 2015a1 {published data only}
-
- Everson G, Cooper C, Hezode C, Shiffman ML, Yoshida E, Beltran‐Jaramillo T, et al. Dauphine: a randomized phase II study of danoprevir/ritonavir plus peginterferon alpha‐2a/ribavirin in HCV genotypes 1 or 4. Liver International 2015;35(1):108‐19. - PubMed
-
- Everson G, Cooper C, Hezode C, Shiffman ML, Yoshida E, Beltran‐Jaramillo T, et al. Rapid and sustained achievement of undetectable HCV RNA during treatment with ritonavir‐boosted danoprevir/peg‐ifna‐2a/RBV in HCV genotype 1 or 4 patients: dauphine week 12 interim analysis. Journal of Hepatology 2012;56(Suppl 2):S466.
-
- Hezode C, Shiffman ML, Cooper C, Everson GT, Marcellin P, Rodriguez‐Torres M, et al. Ritonavir‐boosted danoprevir plus peg‐IFNalpha‐2a/ribavirin (P/R) demonstrates up to 100% SVR24 with 12 or 24 weeks of total treatment in treatment‐naive patients with HCV genotype 4 infection in the DAUPHINE study. Hepatology 2012;56(S1):557A.
-
- Pogam S, Navarro M, Bu L, Voulgari A, Illnicka M, Yan JM, et al. Low rate of on‐treatment resistance to danoprevir boosted by ritonavir (DNVR) combined with Peg‐IFNa‐2a/ribavirin: 12 week interim analysis from Dauphine study. Journal of Hepatology 2012;56(Suppl 2):S472.
Dauphine 2015a2 {published data only}
-
- Everson G, Cooper C, Hezode C, Shiffman ML, Yoshida E, Beltran‐Jaramillo T, et al. Dauphine: a randomized phase II study of danoprevir/ritonavir plus peginterferon alpha‐2a/ribavirin in HCV genotypes 1 or 4. Liver International 2015;35(1):108‐19. - PubMed
-
- Everson G, Cooper C, Hezode C, Shiffman ML, Yoshida E, Beltran‐Jaramillo T, et al. Rapid and sustained achievement of undetectable HCV RNA during treatment with ritonavir‐boosted danoprevir/peg‐ifna‐2a/RBV in HCV genotype 1 or 4 patients: dauphine week 12 interim analysis. Journal of Hepatology 2012;56(Suppl 2):S466.
-
- Hezode C, Shiffman ML, Cooper C, Everson GT, Marcellin P, Rodriguez‐Torres M, et al. Ritonavir‐boosted danoprevir plus peg‐IFNalpha‐2a/ribavirin (P/R) demonstrates up to 100% SVR24 with 12 or 24 weeks of total treatment in treatment‐naive patients with HCV genotype 4 infection in the DAUPHINE study. Hepatology 2012;56(S1):557A.
-
- Pogam S, Navarro M, Bu L, Voulgari A, Illnicka M, Yan JM, et al. Low rate of on‐treatment resistance to danoprevir boosted by ritonavir (DNVR) combined with peg‐IFNa‐2a/ribavirin: 12 week interim analysis from Dauphine study. Journal of Hepatology 2012;56(Suppl 2):S472.
Dauphine 2015a3 {published data only}
-
- Everson G, Cooper C, Hezode C, Shiffman ML, Yoshida E, Beltran‐Jaramillo T, et al. Dauphine: a randomized phase II study of danoprevir/ritonavir plus peginterferon alpha‐2a/ribavirin in HCV genotypes 1 or 4. Liver International 2015;35(1):108‐19. - PubMed
-
- Everson G, Cooper C, Hezode C, Shiffman ML, Yoshida E, Beltran‐Jaramillo T, et al. Rapid and sustained achievement of undetectable HCV RNA during treatment with ritonavir‐boosted danoprevir/peg‐ifna‐2a/RBV in HCV genotype 1 or 4 patients: dauphine week 12 interim analysis. Journal of Hepatology 2012;56(Suppl 2):S466.
-
- Hezode C, Shiffman ML, Cooper C, Everson GT, Marcellin P, Rodriguez‐Torres M, et al. Ritonavir‐boosted danoprevir plus peg‐IFNalpha‐2a/ribavirin (P/R) demonstrates up to 100% SVR24 with 12 or 24 weeks of total treatment in treatment‐naive patients with HCV genotype 4 infection in the DAUPHINE study. Hepatology 2012;56(S1):557A.
-
- Pogam S, Navarro M, Bu L, Voulgari A, Illnicka M, Yan JM, et al. Low rate of on‐treatment resistance to danoprevir boosted by ritonavir (DNVR) combined with peg‐IFNa‐2a/ribavirin: 12 week interim analysis from Dauphine study. Journal of Hepatology 2012;56(Suppl 2):S472.
Dauphine 2015a4 {published data only}
-
- Everson G, Cooper C, Hezode C, Shiffman ML, Yoshida E, Beltran‐Jaramillo T, et al. Dauphine: a randomized phase II study of danoprevir/ritonavir plus peginterferon alpha‐2a/ribavirin in HCV genotypes 1 or 4. Liver International 2015;35(1):108‐19. - PubMed
-
- Everson G, Cooper C, Hezode C, Shiffman ML, Yoshida E, Beltran‐Jaramillo T, et al. Rapid and sustained achievement of undetectable HCV RNA during treatment with ritonavir‐boosted danoprevir/peg‐ifna‐2a/RBV in HCV genotype 1 or 4 patients: dauphine week 12 interim analysis. Journal of Hepatology 2012;56(Suppl 2):S466.
-
- Hezode C, Shiffman ML, Cooper C, Everson GT, Marcellin P, Rodriguez‐Torres M, et al. Ritonavir‐boosted danoprevir plus peg‐IFNalpha‐2a/ribavirin (P/R) demonstrates up to 100% SVR24 with 12 or 24 weeks of total treatment in treatment‐naive patients with HCV genotype 4 infection in the DAUPHINE study. Hepatology 2012;56(S1):557A.
-
- Pogam S, Navarro M, Bu L, Voulgari A, Illnicka M, Yan JM, et al. Low rate of on‐treatment resistance to danoprevir boosted by ritonavir (DNVR) combined with Peg‐IFNa‐2a/ribavirin: 12 week interim analysis from Dauphine study. Journal of Hepatology 2012;56(Suppl 2):S472.
De Bruijne 2010a1 {published data only}
-
- Bruijne J, Bergmann JF, Reesink HW, Weegink CJ, Molenkamp R, Schinkel J, et al. Antiviral activity of narlaprevir combined with ritonavir and pegylated interferon in chronic hepatitis C patients. Hepatology 2010;52(5):1590‐9. - PubMed
-
- Bruijne J, Bergmann JF, Weegink CJ, Molenkamp R, Schinkel J, Treitel MA, et al. Narlaprevir and peginterferon alfa‐2b for 2 weeks in chronic hepatitis C genotype 1 patients, followed by peginterferon alfa‐2b and ribavirin for 24/48 weeks: final results. Journal of Hepatology 2010;52:S290‐1.
-
- Hotho DM, Bruijne J, Spaan M, Treitel MA, Boonstra A, Knegt RJ, et al. Sustained virologic response after therapy with the HCV protease inhibitor narlaprevir in combination with peginterferon and ribavirin is durable through long‐term follow‐up. Journal of Viral Hepatitis 2013;20(4):e78‐81. - PubMed
De Bruijne 2010a2 {published data only}
-
- Bruijne J, Bergmann JF, Reesink HW, Weegink CJ, Molenkamp R, Schinkel J, et al. Antiviral activity of narlaprevir combined with ritonavir and pegylated interferon in chronic hepatitis C patients. Hepatology 2010;52(5):1590‐9. - PubMed
-
- Bruijne J, Bergmann JF, Weegink CJ, Molenkamp R, Schinkel J, Treitel MA, et al. Narlaprevir and peginterferon alfa‐2b for 2 weeks in chronic hepatitis C genotype 1 patients, followed by peginterferon alfa‐2b and ribavirin for 24/48 weeks: final results. Journal of Hepatology 2010;52:S290‐1.
-
- Hotho DM, Bruijne J, Spaan M, Treitel MA, Boonstra A, Knegt RJ, et al. Sustained virologic response after therapy with the HCV protease inhibitor narlaprevir in combination with peginterferon and ribavirin is durable through long‐term follow‐up. Journal of Viral Hepatitis 2013;20(4):e78‐81. - PubMed
Detishin 2011 {published data only}
-
- Detishin V, Haazen W, Hooijmaijers R, Ruppert M, Bol K, Robison H, et al. Final results of the pharmacokinetics, efficacy, and safety/tolerability of 400 and 600 mg once‐daily dosing of ACH‐1625 (HCV NS3 protease inhibitor) in HCV genotype 1. Journal of Hepatology 2011;54(Suppl S1):S186‐7.
Dore 2015a1 {published data only}
-
- Dore GJ, Lawitz E, Hezode C, Shafran SD, Ramji A, Tatum HA, et al. Daclatasvir plus peginterferon and ribavirin is noninferior to peginterferon and ribavirin alone, and reduces the duration of treatment for HCV genotype 2 or 3 infection. Gastroenterology 2015;148(2):355‐66. - PubMed
Dore 2015a2 {published data only}
-
- Dore GJ, Lawitz E, Hezode C, Shafran SD, Ramji A, Tatum HA, et al. Daclatasvir plus peginterferon and ribavirin is noninferior to peginterferon and ribavirin alone, and reduces the duration of treatment for HCV genotype 2 or 3 infection. Gastroenterology 2015;148(2):355‐66. - PubMed
DRAGON 2014a1 {published data only}
DRAGON 2014a2 {published data only}
DRAGON 2014a3 {published data only}
DRAGON 2014a4 {published data only}
Erhardt 2009 {published data only}
-
- Erhardt A, Deterding K, Benhamou Y, Reiser M, Forns X, Pol S, et al. Safety, pharmacokinetics and antiviral effect of BILB 1941, a novel hepatitis C virus RNA polymerase inhibitor, after 5 days oral treatment. Antiviral Therapy 2009;14(1):23‐32. - PubMed
Feld 2014 {published data only}
-
- Feld JJ, Kowdley KV, Coakley E, Sigal S, Nelson D, Crawford D, et al. Sapphire I: Phase 3 placebo‐controlled study of interferon‐free, 12‐week regimen of ABT‐450/R/ABT‐267, ABT‐333, and ribavirin in 631 treatment‐naive adults with hepatitis Cvirus genotype 1. Journal of Hepatology 2014;60(1 Suppl 1):S25.
-
- Feld JJ, Kowdley KV, Coakley E, Sigal S, Nelson DR, Crawford D, et al. Treatment of HCV with ABT‐450/r‐ombitasvir and dasabuvir with ribavirin. New England Journal of Medicine 2014;370(17):1594‐603. - PubMed
-
- Kowdley KV, Feld JJ, Coakley E, Sigal S, Nelson DR, Crawford D, et al. Sapphire I: Phase 3 placebo‐controlled study of interferon‐free, 12‐week regimen of ABT‐450/R/ABT‐267, ABT‐333, and ribavirin in 631 treatment‐naive adults with hepatitis C virus genotype 1. Gastroenterology 2014;146(5 Suppl 1):S912‐S3.
Feld 2015 {published data only}
-
- Feld JJ, Jacobson IM, Hezode C, Asselah T, Ruane PJ, Gruener N, et al. Sofosbuvir and velpatasvir for HCV genotype 1, 2, 4, 5, and 6 infection. New England Journal of Medicine 2015;373(27):2599‐607. - PubMed
FISSION 2013 {published data only}
-
- Lawitz E, Mangia A, Wyles D, Rodriguez‐Torres M, Hassanein T, Gordon Stuart C, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. New England Journal of Medicine 2013;368(20):1878‐87. - PubMed
-
- Younossi ZM, Stepanova M, Henry L, Gane E, Jacobson IM, Lawitz E, et al. Minimal impact of sofosbuvir and ribavirin on health related quality of life in chronic hepatitis C (CH‐C). Journal of Hepatology 2014;60(4):741‐7. [PUBMED: 24333184] - PubMed
Flamm 2013 {published data only}
-
- Flamm S, Lawitz E, Jacobson I, Rubin R, Bourliere M, Hezode C, et al. High sustained virologic response (SVR) among genotype 1 previous non‐responders and relapsers to peginterferon/ribavirin when re‐treated with boceprevir (BOC) plus peginterferon alfa‐2A/ribavirin. Journal of Hepatology 2011;54(Suppl 1):S541‐2.
-
- Flamm SL, Lawitz E, Jacobson I, Bourliere M, Hezode C, Vierling JM, et al. Boceprevir with peginterferon alfa‐2a‐ribavirin is effective for previously treated chronic hepatitis C genotype 1 infection. Clinical Gastroenterology and Hepatology 2013;11(1):81‐7. - PubMed
-
- Howe JA, Ogert RA, Barnard RJ, Hazuda D, Pedicone L, Brass CA. Analysis of sustained viral response and boceprevir resistance following combination treatment with boceprevir plus peginterferon alfa‐2A/ribavirin in HCV genotype 1 prior relapsers and non‐responders. Hepatology 2011;54(S1):443A.
Forestier 2007 {published data only}
-
- Forestier N, Reesink HW, Weegink CJ, McNair L, Kieffer TL, Chu HM, et al. Antiviral activity of telaprevir (VX‐950) and peginterferon alfa‐2a in patients with hepatitis C. Hepatology 2007;46(3):640‐8. - PubMed
-
- Forestier N, Sarrazin C, Friedrich‐Rust M, Weegink C, Jansen P, Reesink H, et al. Status of patients with chronic hepatitis C after a 14‐day pre‐treatment with Telaprevir (VX‐950) followed by an anti‐viral therapy with Peg‐interferon‐alpha‐2a (Peg‐IFN‐2a) and Ribavirin (RBV). Zeitschrift fur Gastroenterologie 2007;45(8):841.
-
- Forestier N, Sarrazin C, Friedrich‐Rust M, Zeuzem S. Status of patients with chronic hepatitis C after a pretreatment period of 14 days with Telaprevir (VX‐950) und a following antiviral therapy with Peg‐interferon‐alfa‐2a (Peg‐IFN‐2s) and ribavirin (RBV). Zeitschrift fur Gastroenterologie 2008;46(1):145.
-
- Forestier N, Weegink CJ, Purdy S, McNair L, Jansen PL, Zeuzem S, et al. Current status of subjects receiving peg‐interferon‐alfa‐2A (peg‐ifn) and ribavirin (RBV) after a 14‐day study of the hepatitis C protease inhibitor telaprevir (VX‐950), with peg‐ifn. Hepatology 2006;44(4 (Suppl 1)):614A‐5A.
-
- Weegink CJ, Forestier N, Jansen PL, Zeuzem S, Reesink HW. Final results of patients receiving peg‐interferon‐alfa‐2a (Peg‐IFN) and ribavirin (RBV) after a 14‐day study of the hepatitis C protease inhibitor telaprevir (VX‐950), with Peg‐IFN. Hepatology 2007;46(4 (Suppl 1)):819A.
Forestier 2011a1 {published data only}
-
- Forestier N, Larrey D, Guyader D, Marcellin P, Rouzier R, Patat A, et al. Treatment of chronic hepatitis C patients with the NS3/4A protease inhibitor danoprevir (ITMN‐191/RG7227) leads to robust reductions in viral RNA: a phase 1b multiple ascending dose study. Journal of Hepatology 2011;54(6):1130‐6. - PubMed
-
- Forestier N, Larrey DG, Guyader D, Marcellin P, Rouzier R, Patat AA, et al. Treatment of chronic hepatitis C virus (HCV) genotype 1 patients with the NS3/4A protease inhibitor ITMN‐191 leads to rapid reductions in plasma HCV RNA: results of a phase 1b multiple ascending dose (MAD) study. Hepatology 2008;48(4 (Suppl)):1132A. - PubMed
-
- Moucari R, Forestier N, Larrey D, Guyader D, Couzigou P, Benhamou Y, et al. Danoprevir, an HCV NS3/4A protease inhibitor, improves insulin sensitivity in patients with genotype 1 chronic hepatitis C. Gut 2010;59(12):1694‐8. - PubMed
Forestier 2011a2 {published data only}
-
- Forestier N, Larrey D, Guyader D, Marcellin P, Rouzier R, Patat A, et al. Treatment of chronic hepatitis C patients with the NS3/4A protease inhibitor danoprevir (ITMN‐191/RG7227) leads to robust reductions in viral RNA: a phase 1b multiple ascending dose study. Journal of Hepatology 2011;54(6):1130‐6. - PubMed
-
- Forestier N, Larrey DG, Guyader D, Marcellin P, Rouzier R, Patat AA, et al. Treatment of chronic hepatitis C virus (HCV) genotype 1 patients with the NS3/4A protease inhibitor ITMN‐191 leads to rapid reductions in plasma HCV RNA: results of a phase 1b multiple ascending dose (MAD) study. Hepatology 2008;48(4 (Suppl)):1132A. - PubMed
-
- Moucari R, Forestier N, Larrey D, Guyader D, Couzigou P, Benhamou Y, et al. Danoprevir, an HCV NS3/4A protease inhibitor, improves insulin sensitivity in patients with genotype 1 chronic hepatitis C. Gut 2010;59(12):1694‐8. - PubMed
Forestier 2011b {published data only}
-
- Forestier N, Larrey D, Marcellin P, Benhamou Y, Guyader D, Bradford W, et al. Antiviral activity and safety of ITMN‐191 in combination with peginterferon alfa‐2a and ribavirin in patients with chronic hepatitis C virus (HCV). Journal of Hepatology 2009;50(Suppl 1):S35.
-
- Forestier N, Larrey D, Marcellin P, Guyader D, Patat A, Rouzier R, et al. Antiviral activity of danoprevir (ITMN‐191/RG7227) in combination with pegylated interferon alpha‐2a and ribavirin in patients with hepatitis C. Journal of Infectious Diseases 2011;204(4):601‐8. - PubMed
Forns 2014 {published data only}
-
- Forns X, Lawitz E, Zeuzem S, Gane E, Bronowicki JP, Andreone P, et al. Simeprevir with peginterferon and ribavirin leads to high rates of SVR in patients with HCV genotype 1 who relapsed after previous therapy: a phase 3 trial. Gastroenterology 2014;146(7):1669‐79. - PubMed
-
- Forns X, Lawitz E, Zeuzem S, Gane EJ, Bronowicki JP, Andreone P, et al. Simeprevir (TMC435) with peg‐interferon alpha‐2a/ribavirin for treatment of chronic HCV genotype 1 infection in patients who relapsed after previous interferon‐based therapy: efficacy and safety in patient sub‐populations in the PROMISE phase III trial. Hepatology 2013;58(4 SUPPL. 1):737A‐8A.
-
- Gane EJ, Forns X, Lawitz E, Zeuzem S, Bronowicki JP, Andreone P, et al. Simeprevir (TMC435) with peg‐interferon alpha‐2a/ribavirin for treatment of chronic HCV genotype 1 infection in patients who relapsed after previous interferon‐based therapy: efficacy in patients with genotype 1b HCV in the PROMISE phase III trial. Hepatology International 2014;8(1 Suppl 1):S181.
-
- Lawitz E, Forns X, Zeuzem S, Gane E, Bronowicki JP, Andreone P, et al. Simeprevir (TMC435) with peginterferon/ribavirin for treatment of chronic HCV genotype 1 infection in patients who relapsed after previous interferon‐based therapy: results from PROMISE, a phase III trial. Gastroenterology 2013;144(5 Suppl 1):S151.
Foster 2011a1 {published data only}
-
- Meyer S, Ghys A, Foster GR, Beumont M, Baelen B, Lin TI, et al. Analysis of genotype 2 and 3 hepatitis C virus variants in patients treated with telaprevir demonstrates a consistent resistance profile across genotypes. Journal of Viral Hepatitis 2013;20(6):395‐403. - PubMed
-
- Meyer S, Ghys A, Foster GR, Beumont‐Mauviel M, Baelen B, Lin TI, et al. Analyses of genotype 2/3 HCV variants in patients treated with telaprevir in study C209 showed that the telaprevir resistance profile appears to be consistent across genotypes. Antiviral Therapy 2010;15(Suppl 2):A118.
-
- Foster GR, Hezode C, Bronowicki JP, Carosi G, Weiland O, Verlinden L, et al. Activity of telaprevir alone or in combination with peginterferon alfa‐2a and ribavirin in treatment‐naive genotype 2 and 3 hepatitis‐C patients: final results of STUDY C209. Journal of Hepatology 2010;52(Suppl 1):S27.
-
- Foster GR, Hézode C, Bronowicki JP, Carosi G, Weiland O, Verlinden L, et al. Telaprevir alone or with peginterferon and ribavirin reduces HCV RNA in patients with chronic genotype 2 but not genotype 3 infections. Gastroenterology 2011;141(3):881‐9. - PubMed
Foster 2011a2 {published data only}
-
- Meyer S, Ghys A, Foster GR, Beumont M, Baelen B, Lin TI, et al. Analysis of genotype 2 and 3 hepatitis C virus variants in patients treated with telaprevir demonstrates a consistent resistance profile across genotypes. Journal of Viral Hepatitis 2013;20(6):395‐403. - PubMed
-
- Meyer S, Ghys A, Foster GR, Beumont‐Mauviel M, Baelen B, Lin TI, et al. Analyses of genotype 2/3 HCV variants in patients treated with telaprevir in study C209 showed that the telaprevir resistance profile appears to be consistent across genotypes. Antiviral Therapy 2010;15(Suppl 2):A118.
-
- Foster GR, Hezode C, Bronowicki JP, Carosi G, Weiland O, Verlinden L, et al. Activity of telaprevir alone or in combination with peginterferon alfa‐2a and ribavirin in treatment‐naive genotype 2 and 3 hepatitis‐C patients: final results of STUDY C209. Journal of Hepatology 2010;52(Suppl 1):S27.
-
- Foster GR, Hézode C, Bronowicki JP, Carosi G, Weiland O, Verlinden L, et al. Telaprevir alone or with peginterferon and ribavirin reduces HCV RNA in patients with chronic genotype 2 but not genotype 3 infections. Gastroenterology 2011;141(3):881‐9. - PubMed
Foster 2015a1 {published data only}
-
- Foster GR, Afdhal N, Roberts SK, Brau N, Gane EJ, Pianko S, et al. Sofosbuvir and velpatasvir for HCV genotype 2 and 3 infection. New England Journal of Medicine 2015;373(27):2608‐17. - PubMed
Foster 2015a2 {published data only}
-
- Foster GR, Afdhal N, Roberts SK, Brau N, Gane EJ, Pianko S, et al. Sofosbuvir and velpatasvir for HCV genotype 2 and 3 infection. New England Journal of Medicine 2015;373(27):2608‐17. - PubMed
Fried 2013 {published data only}
-
- Aerssens J, Fanning G, Scholliers A, Lenz O, Peeters M, Smedt G, et al. Impact of IL28B genotype and pretreatment serum IP‐10 in treatment‐naive genotype‐1 HCV patients treated with TMC435 in combination with peginterferona‐2A and ribavirin in PILLAR study. Journal of Hepatology 2011;54(Suppl S1):S5‐6.
-
- Fried MW, Buti M, Dore GJ, Ferenci P, Jacobson I, Marcellin P, et al. Efficacy and safety of TMC435 in combination with peginterferon alfa‐2a and ribavirin in treatment‐naive genotype‐1 HCV patients: 24‐week interim results from the PILLAR study. Hepatology 2010;52(Suppl S1):403A.
-
- Scott J, Gilles L, Fu M, Brohan E, Amatya R, Jessner W, et al. Patients with chronic hepatitis C virus treated with simeprevir added to peginterferon and ribavirin experienced less time with fatigue, depressive symptoms, and functional limitations: results from patients in the QUEST‐1, QUEST‐2, and promise studies. Value in Health 2013;16(7):A362. - PubMed
-
- Scott J, Gilles L, Fu M, Brohan E, Panter C, Arbuckle R, et al. Simeprevir added to peginterferon and ribavirin lessens time with fatigue, depressive symptoms and functional limitations in patients with chronic hepatitis C compared with peginterferon and ribavirin: results from 1161 patients in the QUEST‐1, QUEST‐2 and PROMISE studies. Journal of Viral Hepatitis 2015;22(8):639‐50. - PubMed
Fundamental 2014a1 {published data only}
-
- Buti M, Flisiak R, Kao JH, Chuang WL, Streinu‐Cercel A, Tabak F, et al. Alisporivir with peginterferon/ribavirin in patients with chronic hepatitis C genotype 1 infection who failed to respond to or relapsed after prior interferon‐based therapy: FUNDAMENTAL, a phase II trial. Journal of Viral Hepatitis 2014;22(7):596‐606. [DOI: 10.1111/jvh.12360] - DOI - PubMed
-
- Chuang WL, Kao JH, Sheen IS, Hsu SJ, Hung CH, Zekry A, et al. Superior SVR24 rates with alisporivir (ALV) plus peg‐interferon/ribavirin (P/R) in chronic HCV hepatitis (CHC) genotype 1 (G1) prior P/R treatment failures: final results of the fundamental study. Hepatology International 2014;8(1 Suppl 1):S206.
Fundamental 2014a2 {published data only}
-
- Buti M, Flisiak R, Kao JH, Chuang WL, Streinu‐Cercel A, Tabak F, et al. Alisporivir with peginterferon/ribavirin in patients with chronic hepatitis C genotype 1 infection who failed to respond to or relapsed after prior interferon‐based therapy: FUNDAMENTAL, a phase II trial. Journal of Viral Hepatitis 2014;22(7):596‐606. [DOI: 10.1111/jvh.12360] - DOI - PubMed
-
- Chuang WL, Kao JH, Sheen IS, Hsu SJ, Hung CH, Zekry A, et al. Superior SVR24 rates with alisporivir (ALV) plus peg‐interferon/ribavirin (P/R) in chronic HCV hepatitis (CHC) genotype 1 (G1) prior P/R treatment failures: Final results of the fundamental study. Hepatology International 2014;8(1 Suppl 1):S206.
Fundamental 2014a3 {published data only}
-
- Buti M, Flisiak R, Kao JH, Chuang WL, Streinu‐Cercel A, Tabak F, et al. Alisporivir with peginterferon/ribavirin in patients with chronic hepatitis C genotype 1 infection who failed to respond to or relapsed after prior interferon‐based therapy: FUNDAMENTAL, a phase II trial. Journal of Viral Hepatitis 2014;22(7):596‐606. [DOI: 10.1111/jvh.12360] - DOI - PubMed
-
- Chuang WL, Kao JH, Sheen IS, Hsu SJ, Hung CH, Zekry A, et al. Superior SVR24 rates with alisporivir (ALV) plus peg‐interferon/ribavirin (P/R) in chronic HCV hepatitis (CHC) genotype 1 (G1) prior P/R treatment failures: Final results of the fundamental study. Hepatology International 2014;8(1 Suppl 1):S206.
Gane 2008 {published data only}
-
- Gane EJ, Rodriguez‐Torres M, Nelson DR, Jacobson IM, McHutchison JG, Jeffers L, et al. Antiviral activity of the HCV nucleoside polymerase inhibitor R7128 in HCV genotype 2 and 3 prior non‐responders: interim results of R7128 1500mg BID with PEG‐IFN and ribavirin for 28 days. Hepatology 2008;48(4 Suppl):1024A.
-
- Lalezari J, Gane E, Rodriguez‐Torres M, DeJesus E, Nelson D, Everson G, et al. Potent antiviral activity of the HCV nucleoside polymerase inhibitor R7128 with peg‐IFN and ribavirin: interim results of R7128 500MG bid for 28 days. Journal of Hepatology 2008;48(Suppl 2):S29.
Gane 2010 {published data only}
-
- Chu T, Kulkarni R, Gane EJ, Roberts SK, Stedman C, Angus P, et al. The effect of host IL28B genotype on early viral kinetics during interferon‐free treatment in patients with chronic hepatitis C (CHC). Journal of Hepatology 2011;54(Suppl 1):S521‐2. - PubMed
-
- Gane EJ, Roberts SK, Stedman C, Angus PW, Ritchie B, Elston R, et al. First‐in‐man demonstration of potent antiviral activity with a nucleoside polymerase (R7128) and protease (R7227/ITMN‐191) inhibitor combination in HCV: safety, pharmacokinetics, and virologic results from INFORM‐1. Journal of Hepatology 2009;50(Suppl 1):S380.
-
- Gane EJ, Roberts SK, Stedman CA, Angus PW, Ritchie B, Elston R, et al. Combination therapy with a nucleoside polymerase (R7128) and protease (R7227/ITMN ‐ 191) inhibitor in HCV: safety, pharmacokinetics, and virologic results from INFORM‐1. Hepatology 2009;50(4 Suppl):394A‐5A.
-
- Gane EJ, Roberts SK, Stedman CAM, Angus PW, Ritchie B, Elston ROB, et al. Oral combination therapy with a nucleoside polymerase inhibitor (RG7128) and danoprevir for chronic hepatitis C genotype 1 infection (INFORM‐1): a randomised, double‐blind, placebo‐controlled, dose‐escalation trial. Lancet 2010;376(9751):1467‐75. - PubMed
-
- Pogam S, Yan JM, Chhabra M, Ilnicka M, Kang H, Kosaka A, et al. Characterization of hepatitis C virus (HCV) quasispecies dynamics upon short‐term dual therapy with the HCV NS5B nucleoside polymerase inhibitor mericitabine and the NS3/4 protease inhibitor danoprevir. Antimicrobial Agents and Chemotherapy 2012;56(11):5494‐502. - PMC - PubMed
Gane 2011 {published data only}
-
- Gane E, Rouzier R, Stedman C, Wiercinska‐Drapalo A, Horban A, Chang L, et al. Ritonavir boosting of low dose RG7227/ITMN‐191, HCV NS3/4 a protease inhibitor, results in robust reduction in HCV RNA at lower exposures than provided by unboosted regimens. Journal of Hepatology 2010;52(Suppl 1):S16‐7.
-
- Gane EJ, Rouzier R, Stedman C, Wiercinska‐Drapalo A, Horban A, Chang L, et al. Antiviral activity, safety, and pharmacokinetics of danoprevir/ritonavir plus PEG‐IFN alpha‐2a/RBV in hepatitis C patients. Journal of Hepatology 2011;55(5):972‐9. - PubMed
-
- Morcos PN, Chang L, Kulkarni R, Giraudon M, Shulman N, Brennan BJ, et al. A randomised study of the effect of danoprevir/ritonavir or ritonavir on substrates of cytochrome P450 (CYP) 3A and 2C9 in chronic hepatitis C patients using a drug cocktail. European Journal of Clinical Pharmacology 2013;69(11):1939‐49. - PubMed
Gane 2015 {published data only}
-
- Gane E, Schwabe C, Mader M, Suri V, Donohue M, Huang M, et al. Sustained virologic response after ACH‐3102 and sofosbuvir treatment for 8 or 6 weeks: A phase 2 "proxy" study. Journal of Hepatology 2015;62(Suppl S2):S266.
Gardner 2014a {published data only}
-
- Gardner S, Cutrell AMY, Elko‐Simms C, Adkison K, Hamatake R, Walker J, et al. A double‐blind, randomized, placebo‐controlled study to assess the safety, antiviral activity and pharmacokinetics of GSK2336805 when given as monotherapy and in combination with peginterferon alfa‐2a and ribavirin in hepatitis C virus genotype 1‐infected treatment‐naive subjects. Liver International 2014;34(6):e89‐e95. - PubMed
GlaxoSmithKline 2014 {published data only}
-
- GlaxoSmithKline plc. A randomized, single blind, dose escalation, placebo‐controlled study to assess the safety, pharmacokinetics, and antiviral activity of repeat doses of GSK2878175 in subjects with chronic hepatitis C. www.gsk‐clinicalstudyregister.com/files2/116976‐Clinical‐Study‐Result‐Summary.pdf (accessed 25 January 2016).
Goldwater 2010 {published data only}
-
- Goldwater R, DeMicco MP, Zong J, Chittick GE, Yuen GJ, West S, et al. Safety, pharmacokinetics, and antiviral activity of single oral doses of the HCV NS3 protease inhibitor GS 9256. Hepatology 2010;52(Suppl S1):717A.
HALLMARK‐DUAL 2014 {published data only}
-
- Jacobson I, Kumada H, Chayama K, Dore G, Pol S, Zeuzem S, et al. Safety and tolerability of daclatasvir (DCV) in patients with chronic HCV infection. Hepatology International 2015;9(1 Suppl):S278‐9.
-
- Manns M, Pol S, Jacobson IM, Marcellin P, Gordon SC, Peng CY, et al. All‐oral daclatasvir plus asunaprevir for hepatitis C virus genotype 1b: a multinational, phase 3, multi cohort study. Lancet 2014;384(9954):1597‐605. - PubMed
Han 2014 {published data only}
-
- Han KH, Helmond FA, Paik SW, Han SY, Heo J, Tak WY. Boceprevir plus peginterferon alfa and ribavirin for Korean patients with chronic hepatitis C virus genotype 1 infection and previous treatment failure. Hepatology International 2014;8(1 Suppl 1):S222‐3.
Hezode 2009 {published data only}
-
- Bronowicki JP, Hezode C, Bengtsson L, Pol S, Bourliere M, Serfaty L, et al. 100% SVR in IL28B CC patients treated with 12 weeks of telaprevir, peginterferon and ribavirin in the PROVE2 trial. Journal of Hepatology 2012;56(Suppl 2):S430‐1.
-
- Dusheiko GM, Hezode C, Pol S, Goeser T, Bronowicki JP, Bourliere M, et al. Treatment of chronic hepatitis C with telaprevir (TVR) in combination with peginterferon‐alfa‐2a with or without ribavirin: further interim analysis results of the PROVE2 study. Journal of Hepatology 2008;48(Suppl 2):S26.
-
- Hezode C, Ferenci P, Dusheiko Geoffrey M, Tran A, Grange J‐D, Mathurin P, et al. Prove2 Study: treatment of chronic hepatitis C with telaprevir (Tvr) in combination with peginterferon‐alfa‐2a with or without ribavirin, interim analysis results. Gastroenterology 2008;134(4, Suppl. 1):A755.
-
- Hezode C, Forestier N, Dusheiko G, Ferenci P, Pol S, Goeser T, et al. Telaprevir and peginterferon with or without ribavirin for chronic HCV infection. New England Journal of Medicine 2009;360(18):1839‐50. - PubMed
-
- Muir A, Poordad F, Sheikh A, Elkashab M, Brennan R, Ankoma‐Sey V, et al. SVR4 results for the combination of ACH‐3102 and sovaprevir, with ribavirin, in subjects with genotype 1 chronic hepatitis C infection. Hepatology International 2014;8(1 Suppl):s395.
Hinrichsen 2004 {published data only}
-
- Hinrichsen H, Benhamou Y, Wedemeyer H, Reiser M, Sentjens RE, Calleja JL, et al. Short‐term antiviral efficacy of BILN 2061, a hepatitis C virus serine protease inhibitor, in hepatitis C genotype 1 patients. Gastroenterology 2004;127:1347–55. - PubMed
-
- Wedemeyer H, Erhardt A, Schmiegel W, Hinrichsen H, Chaves R, Yong CL, et al. Safety and antiviral effect of BILN 2061, a novel HCV serine protease inhibitor, after oral treatment over 2 days in patients with chronic hepatitis C, genotype 1, and liver cirrhosis. Hepatology 2003;38(4 Suppl 1):297A.
Hoeben 2015a1 {published data only}
-
- Hoeben E, Viberg A, Petersson K, Lee M, Vanwelkenhuysen I, Witek J, et al. Simeprevir exposure in Asian treatment naive patients with chronic hepatitis C virus genotype 1 infection results from a population pharmacokinetic model in the phase III Tiger study. Hepatology International 2015;9(1 Suppl):S71.
-
- Wei L, Han T, Yang D, Heo J, Shang J, Cheng J. Simeprevir plus peginterferon ribavirin in treatment naive patients with chronic hepatitis C virus genotype 1 infection results from the phase III Tiger study conducted in East Asian patients living in China and Korea. Hepatology International 2015;9(1 Suppl):S61.
Hoeben 2015a2 {published data only}
-
- Hoeben E, Viberg A, Petersson K, Lee M, Vanwelkenhuysen I, Witek J, et al. Simeprevir exposure in Asian treatment naive patients with chronic hepatitis C virus genotype 1 infection results from a population pharmacokinetic model in the phase III Tiger study. Hepatology International 2015;9(1 Suppl):S71.
-
- Wei L, Han T, Yang D, Heo J, Shang J, Cheng J. Simeprevir plus peginterferon ribavirin in treatment naive patients with chronic hepatitis C virus genotype 1 infection results from the phase III Tiger study conducted in East Asian patients living in China and Korea. Hepatology International 2015;9(1 Suppl):S61.
Hotho 2012 {published data only}
-
- Hotho D, Bruijne J, O'Farrell A, Boyea T, Li J, Weegink CJ, et al. Accelerated clinical trial design to assess the safety, tolerability and anti‐viral activity of PHX1766, a novel HCV NS3/4A protease inhibitor, in healthy volunteers and chronic hepatitis C patients. Hepatology 2009;50(4 Suppl):1031A‐2A.
-
- Hotho Daphne M, Bruijne J, O'Farrell A, Boyea T, Li J, Bracken M, et al. Pharmacokinetics and antiviral activity of PHX1766, a novel HCV protease inhibitor, using an accelerated Phase I study design. Antiviral Therapy 2012;17(2):365‐75. - PubMed
Isakov 2016 {published data only}
Izumi 2014a1 {published data only}
-
- Izumi N, Asahina Y, Yokosuka O, Imazeki F, Kawada N, Tamori A, et al. Combination therapy of treatment‐naive and nonresponder patients with HCV genotype 1 infection with BMS‐790052, an NS5A replication complex inhibitor, in combination with peginterferon alfa‐2a and ribavirin. Hepatology 2011;54(S1):1439A‐40A.
-
- Izumi N, Yokosuka O, Kawada N, Osaki Y, Yamamoto K, Sata M, et al. Daclatasvir combined with peginterferon alfa‐2a and ribavirin in Japanese patients infected with hepatitis C genotype 1. Antiviral Therapy 2014;19(5):501‐10. - PubMed
-
- McPhee F, Hernandez D, Zhou N, Yu F, Ueland J, Monikowski A, et al. Virological escape in HCV genotype‐1‐infected patients receiving daclatasvir plus ribavirin and peginterferon alfa‐2a or alfa‐2b. Antiviral Therapy 2014;19(5):479‐90. - PubMed
-
- Suzuki F, Chayama K, Kawakami Y, Toyota J, Karino Y, Mochida S, et al. BMS‐790052, AN NS5A replication complex inhibitor, in combination with peginterferon alpha‐2B and ribavirin in Japanese treatment naive and nonresponder patients with chronic HCV genotype 1 infection. Hepatology 2011;54(S1):1441A.
-
- Suzuki F, Chayama K, Kawakami Y, Toyota J, Karino Y, Mochida S, et al. Daclatasvir (BMS‐790052), an NS5A replication complex inhibitor, in combination with peginterferon alpha‐2b and ribavirin in Japanese treatment‐naive and nonresponder patients with chronic HCV genotype 1 infection. Hepatology International 2012;6(1):161‐2.
Izumi 2014a2 {published data only}
-
- Izumi N, Asahina Y, Yokosuka O, Imazeki F, Kawada N, Tamori A, et al. Combination therapy of treatment‐naive and nonresponder patients with HCV genotype 1 infection with BMS‐790052, an NS5A replication complex inhibitor, in combination with peginterferon alfa‐2a and ribavirin. Hepatology 2011;54(S1):1439A‐40A.
-
- Izumi N, Yokosuka O, Kawada N, Osaki Y, Yamamoto K, Sata M, et al. Daclatasvir combined with peginterferon alfa‐2a and ribavirin in Japanese patients infected with hepatitis C genotype 1. Antiviral Therapy 2014;19(5):501‐10. - PubMed
-
- McPhee F, Hernandez D, Zhou N, Yu F, Ueland J, Monikowski A, et al. Virological escape in HCV genotype‐1‐infected patients receiving daclatasvir plus ribavirin and peginterferon alfa‐2a or alfa‐2b. Antiviral Therapy 2014;19(5):479‐90. - PubMed
-
- Suzuki F, Chayama K, Kawakami Y, Toyota J, Karino Y, Mochida S, et al. BMS‐790052, AN NS5A replication complex inhibitor, in combination with peginterferon alpha‐2B and ribavirin in Japanese treatment naive and nonresponder patients with chronic HCV genotype 1 infection. Hepatology 2011;54(S1):1441A.
-
- Suzuki F, Chayama K, Kawakami Y, Toyota J, Karino Y, Mochida S, et al. Daclatasvir (BMS‐790052), an NS5A replication complex inhibitor, in combination with peginterferon alpha‐2b and ribavirin in Japanese treatment‐naive and nonresponder patients with chronic HCV genotype 1 infection. Hepatology International 2012;6(1):161‐2.
Jacobson 2010 {published data only}
-
- Jacobson I, Pockros P, Lalezari J, Lawitz E, Rodriguez‐Torres M, DeJesus E, et al. Antiviral activity of filibuvir in combination with pegylated interferon alfa‐2A and ribavirin for 28 days in treatment naive patients chronically infected with HCV genotype 1. Journal of Hepatology 2009;50(Suppl 1):S382.
-
- Jacobson I, Pockros PJ, Lalezari J, Lawitz E, Rodriguez‐Torres M, DeJesus E, et al. Virologic response rates following 4 weeks of filibuvir in combination with pegylated interferon alfa‐2A and ribavirin in chronically‐infected HCV genotype‐1 patients. Journal of Hepatology 2010;52(Suppl 1):S464.
-
- Mori J, Hammond JL, Srinivasan S, Jagannatha S, Ryst ECC. Genotypic characterisation of filibuvir (PF‐00868554 ) resistance in patients receiving four weeks co‐administration of filibuvir with pegIFN/RBV. Hepatology 2010;52(Suppl S1):722A.
-
- Mori J, Hammond JL, Srinivasan S, Jagannatha S, Ryst E. Genotypic characterisation of filibuvir (PF‐00868554) resistance in patients receiving four weeks co‐administration of filibuvir with PEGIFN/RBV (12 week analysis). Journal of Hepatology 2010;52(Suppl 1):S15.
Jacobson 2014 {published data only}
-
- Jacobson I, Dore GJ, Foster GR, Fried MW, Radu M, Rafalskiy VV, et al. Simeprevir (TMC435) with peginterferon/ribavirin for chronic HCV genotype‐1 infection in treatment‐naive patients: results from QUEST‐1, a phase III trial. Journal of Hepatology 2013;58(Suppl 1):S574.
-
- Jacobson IM, Dore GJ, Foster GR, Fried MW, Radu M, Rafalsky VV, et al. Simeprevir with pegylated interferon alfa 2a plus ribavirin in treatment‐naive patients with chronic hepatitis C virus genotype 1 infection (QUEST‐1): a phase 3, randomised, double‐blind, placebo‐controlled trial. Lancet 2014;384(9941):403‐13. - PubMed
JUMP‐C 2013 {published data only}
-
- Pockros PJ, Jensen D, Tsai N, Taylor R, Ramji A, Cooper C, et al. JUMP‐C: a randomized trial of mericitabine plus pegylated interferon alpha‐2a/ribavirin for 24 weeks in treatment‐naïve HCV genotype 1/4 patients. Hepatology 2013;58(2):514‐23. - PubMed
Kwo 2010a1 {published data only}
-
- Kwo P, Lawitz E, McCone J, Schiff E, Vierling J, Pound D, et al. HCV Sprint‐1 final results: SVR 24 from a phase 2 study of boceprevir plus pegintron (peginterferon ALFA‐2B)/ribavirin in treatment‐naive subjects with genotype‐1 chronic hepatitis C. Journal of Hepatology 2009;50(Suppl 1):S4.
-
- Kwo PJ, Lawitz EJ, McCone J, Schiff ER, Vierling JM, Pound D, et al. Efficacy of boceprevir, an NS3 protease inhibitor, in combination with peginterferon alfa‐2b and ribavirin in treatment‐naive patients with genotype 1 hepatitis C infection (SPRINT‐1): an open‐label, randomised, multicentre phase 2 trial. Lancet 2010;376(9742):705‐16. - PubMed
-
- Ogert RA, Howe JA, Vierling JM, Kwo PY, Lawitz EJ, McCone J, et al. Resistance‐associated amino acid variants associated with boceprevir plus pegylated interferon‐alpha2b and ribavirin in patients with chronic hepatitis C in the SPRINT‐1 trial. Antiviral Therapy 2013;18(3):387‐97. - PubMed
-
- Poordad F, Vierling JM, Esteban R, Kwo PY, Long J, Chaudhri EI, et al. Hemogloblin decline during lead‐in phase as an early predictor of anemia after the addition of boceprevir: a retrospective analysis of HCV SPRINT‐1. Hepatology 2010;52(Suppl S1):770A.
-
- Vierling JM, Kwo PY, Lawitz E, McCone J, Schiff ER, Pound D, et al. Frequencies of resistance‐associated amino acid variants following combination treatment with boceprevir plus PEGINTRON (peginterferon alfa‐2b)/ribavirin in patients with chronic hepatitis C (CHC), genotype 1 (G1). Hepatology 2010;52(Suppl S1):702A.
Kwo 2010a2 {published data only}
-
- Kwo P, Lawitz E, McCone J, Schiff E, Vierling J, Pound D, et al. HCV sprint‐1 final results: SVR 24 from a phase 2 study of boceprevir plus pegintron (peginterferon ALFA‐2B)/ribavirin in treatment‐naive subjects with genotype‐1 chronic hepatitis C. Journal of Hepatology 2009;50(Suppl 1):S4.
-
- Kwo PJ, Lawitz EJ, McCone J, Schiff ER, Vierling JM, Pound D, et al. Efficacy of boceprevir, an NS3 protease inhibitor, in combination with peginterferon alfa‐2b and ribavirin in treatment‐naive patients with genotype 1 hepatitis C infection (SPRINT‐1): an open‐label, randomised, multicentre phase 2 trial. Lancet 2010;376(9742):705‐16. - PubMed
-
- Ogert RA, Howe JA, Vierling JM, Kwo PY, Lawitz EJ, McCone J, et al. Resistance‐associated amino acid variants associated with boceprevir plus pegylated interferon‐alpha2b and ribavirin in patients with chronic hepatitis C in the SPRINT‐1 trial. Antiviral Therapy 2013;18(3):387‐97. - PubMed
-
- Poordad F, Vierling JM, Esteban R, Kwo PY, Long J, Chaudhri EI, et al. Hemogloblin decline during lead‐in phase as an early predictor of anemia after the addition of boceprevir: a retrospective analysis of HCV SPRINT‐1. Hepatology 2010;52(Suppl S1):770A.
-
- Vierling JM, Kwo PY, Lawitz E, McCone J, Schiff ER, Pound D, et al. Frequencies of resistance‐associated amino acid variants following combination treatment with boceprevir plus PEGINTRON (peginterferon alfa‐2b)/ribavirin in patients with chronic hepatitis C (CHC), genotype 1 (G1). Hepatology 2010;52(Suppl S1):702A.
Kwo 2010a3 {published data only}
-
- Kwo P, Lawitz E, McCone J, Schiff E, Vierling J, Pound D, et al. HCV Sprint‐1 final results: SVR 24 from a phase 2 study of boceprevir plus pegintron (peginterferon ALFA‐2B)/ribavirin in treatment‐naive subjects with genotype‐1 chronic hepatitis C. Journal of Hepatology 2009;50(Suppl 1):S4.
-
- Kwo PJ, Lawitz EJ, McCone J, Schiff ER, Vierling JM, Pound D, et al. Efficacy of boceprevir, an NS3 protease inhibitor, in combination with peginterferon alfa‐2b and ribavirin in treatment‐naive patients with genotype 1 hepatitis C infection (SPRINT‐1): an open‐label, randomised, multicentre phase 2 trial. Lancet 2010;376(9742):705‐16. - PubMed
-
- Ogert RA, Howe JA, Vierling JM, Kwo PY, Lawitz EJ, McCone J, et al. Resistance‐associated amino acid variants associated with boceprevir plus pegylated interferon‐alpha2b and ribavirin in patients with chronic hepatitis C in the SPRINT‐1 trial. Antiviral Therapy 2013;18(3):387‐97. - PubMed
-
- Poordad F, Vierling JM, Esteban R, Kwo PY, Long J, Chaudhri EI, et al. Hemogloblin decline during lead‐in phase as an early predictor of anemia after the addition of boceprevir: a retrospective analysis of HCV SPRINT‐1. Hepatology 2010;52(Suppl S1):770A.
-
- Vierling JM, Kwo PY, Lawitz E, McCone J, Schiff ER, Pound D, et al. Frequencies of resistance‐associated amino acid variants following combination treatment with boceprevir plus PEGINTRON (peginterferon alfa‐2b)/ribavirin in patients with chronic hepatitis C (CHC), genotype 1 (G1). Hepatology 2010;52(Suppl S1):702A.
Kwo 2010a4 {published data only}
-
- Kwo P, Lawitz E, McCone J, Schiff E, Vierling J, Pound D, et al. HCV Sprint‐1 final results: SVR 24 from a phase 2 study of boceprevir plus pegintron (peginterferon ALFA‐2B)/ribavirin in treatment‐naive subjects with genotype‐1 chronic hepatitis C. Journal of Hepatology 2009;50(Suppl 1):S4.
-
- Kwo PJ, Lawitz EJ, McCone J, Schiff ER, Vierling JM, Pound D, et al. Efficacy of boceprevir, an NS3 protease inhibitor, in combination with peginterferon alfa‐2b and ribavirin in treatment‐naive patients with genotype 1 hepatitis C infection (SPRINT‐1): an open‐label, randomised, multicentre phase 2 trial. Lancet 2010;376(9742):705‐16. - PubMed
-
- Ogert RA, Howe JA, Vierling JM, Kwo PY, Lawitz EJ, McCone J, et al. Resistance‐associated amino acid variants associated with boceprevir plus pegylated interferon‐alpha2b and ribavirin in patients with chronic hepatitis C in the SPRINT‐1 trial. Antiviral Therapy 2013;18(3):387‐97. - PubMed
-
- Poordad F, Vierling JM, Esteban R, Kwo PY, Long J, Chaudhri EI, et al. Hemogloblin decline during lead‐in phase as an early predictor of anemia after the addition of boceprevir: a retrospective analysis of HCV SPRINT‐1. Hepatology 2010;52(Suppl S1):770A.
-
- Vierling JM, Kwo PY, Lawitz E, McCone J, Schiff ER, Pound D, et al. Frequencies of resistance‐associated amino acid variants following combination treatment with boceprevir plus PEGINTRON (peginterferon alfa‐2b)/ribavirin in patients with chronic hepatitis C (CHC), genotype 1 (G1). Hepatology 2010;52(Suppl S1):702A.
Lalezari 2011 {published data only}
-
- Lalezari JP, Hazan L, Kankam M, Lawitz E, Poordad FF, Araya V, et al. High rapid virologic response (RVR) with ach‐1625 daily dosing plus pegifn‐alpha 2a/RBV in a 28‐day phase 2a trial. Hepatology 2011;54(Suppl S1):992A‐3A.
Lalezari 2012 {published data only}
Lalezari 2013 {published data only}
-
- Lalezari J, Box T, O'Riordan W, Mehra P, Nguyen T, Poordad F, et al. IDX184 in combination with pegylated interferon‐alpha2a and ribavirin for 2 weeks in treatment‐naive patients with chronic hepatitis C. Antiviral Therapy 2013;18(6):755‐64. - PubMed
-
- Lalezari J, Poordad F, Mehra P, Nguyen T, Dejesus E, Godofsky E, et al. Antiviral activity, pharmacokinetics and safety of IDX184 in combination with pegylated interferon (pegIFN) and ribavirin (RBV) in treatment‐naive HCV genotype 1‐infected subjects. Journal of Hepatology 2010;52(Suppl S1):S469.
-
- Lalezari JP, O'Riordan W, Poordad F, Nguyen TT, Patrick GD, Chen J, et al. A phase IIA study of IDX184 in combination with pegylated interferon (PEGIFN) and ribavirin (RBV) in treatment‐naive HCV genotype 1‐infected subjects. Hepatology 2010;52(Suppl S1):337A.
Larrey 2012 {published data only}
-
- Larrey D, Lohse A, Ledinghen V, Trepo C, Gerlach T, Zarski JP, et al. 4 week therapy with the non‐nucleosidic polymerase inhibitor BI207127 in combination with peginterferon‐alfa2A and ribavirin in treatment naive and treatment experienced chronic HCV GT1 patients. Journal of Hepatology 2010;52(Suppl S1):S466.
-
- Larrey D, Lohse AW, Ledinghen V, Trepo C, Gerlach T, Zarski JP, et al. Rapid and strong antiviral activity of the non‐nucleosidic NS5B polymerase inhibitor BI 207127 in combination with peginterferon alfa 2a and ribavirin. Journal of Hepatology 2012;57(1):39‐46. - PubMed
Larrey 2013 {published data only}
-
- Lagace L, Cartier M, Laflamme G, Lawetz C, Marquis M Triki I, et al. Genotypic and phenotypic analysis of the NS5B polymerase region from viral isolates of HCV chronically infected patients treated with BI 207127 for 5‐days monotherapy. Hepatology 2010;52(Suppl S1):1205A‐6A.
-
- Larrey D, Lohse AW, Trepo C, Bronowicki JP, Arasteh K, Bourliere M, et al. Antiviral effect, safety, and pharmacokinetics of five‐day oral administration of deleobuvir (BI 207127), an investigational hepatitis C virus RNA polymerase inhibitor, in patients with chronic hepatitis C. Antimicrobial Agents and Chemotherapy 2013;57(10):4727‐35. - PMC - PubMed
-
- Larrey DG, Benhamou Y, Lohse AW, Trepo C, Moelleken C, Bronowicki J, et al. BI 207127 is a potent HCV RNA polymerase inhibitor during 5 days monotherapy in patients with chronic hepatitis C. Hepatology 2009;50(4):1044A.
Lawitz 2008 {published data only}
-
- Lawitz EJ, Sulkowski MS, Jacobson IM, Faruqui S, Kraft WK, Maliakkal B, et al. Safety, tolerability and antiviral activity of MK‐7009, a novel inhibitor of the hepatitis C virus NS3/4A protease, in patients with chronic HCV genotype 1 infection. Hepatology 2008;48(4 Suppl):403A‐4A.
Lawitz 2009 {published data only}
-
- Lawitz E, Cooper C, Rodriguez‐Torres M, Ghalib R, Lalonde R, Sheikh A, et al. Safety, tolerability and antiviral activity of VCH‐916, a novel non‐nucleoside HCV polymerase inhibitor in patients with chronic HCV genotype‐1 infection. Journal of Hepatology 2009;50(Suppl S1):S37.
Lawitz 2010a {published data only}
-
- Lawitz EJ, Marbury TC, Vince BD, Grunenberg N, Rodriguez‐Torres M, Micco MP, et al. Dose‐ranging, three‐day monotherapy study of the HCV NS3 protease inhibitor GS‐9256. Journal of Hepatology 2010;52(Suppl 1):S466‐S7.
Lawitz 2010b {published data only}
-
- Lawitz E, Lalezari JP, Rodriguez‐Torres M, Kowdley KV, Nelson D, DeJesus E, et al. Clinical synergy of an anti‐HCV nucleoside analog with SOC: viral kinetics of PSI‐7977 with SOC. Hepatology 2010;52(Suppl S1):1205A.
Lawitz 2010c {published data only}
-
- Lawitz E, Rodriguez‐Torres M, Rustgi VK, Hassanein T, Rahimy MH, Crowley CA, et al. Safety and antiviral activity of ANA598 in combination with pegylated interferon alpha2A plus ribavirin in treatment‐naive genotype‐1 chronic HCV patients. Hepatology 2010;52(Suppl S1):334A‐5A.
-
- Lawitz E, Rodriquez‐Torres M, Rustgi VK, Hassanein T, Rahimy MH, Crowley CA, et al. Safety and antiviral activity of ANA598 in combination with pegylated interferon alpha2a plus ribavirin in treatment‐naive genotype‐1 chronic HCV patients. Journal of Hepatology 2010;52(Suppl S1):S467.
-
- Muir AJ, Lawitz E, Rodriguez‐Torres M, Rustgi VK, Hassanein T, Appleman JR, et al. IL28B polymorphism and kinetics of antiviral activity for ANA598 in combination with pegylated interferon α2A plus ribavirin in treatment‐naïve genotype‐1 chronic HCV patients. Hepatology 2010;52(Suppl S1):1200A.
Lawitz 2011a {published data only}
-
- Lawitz E, Gaultier I, Poordad F, Cohen DE, Menon R. ABT‐450/ritonavir (ABT‐450/R) combined with pegylated interferon alpha‐2a and ribavirin (SOC) after 3‐day monotherapy in genotype 1 HCV‐infected treatment‐naive subjects: 12‐week interim efficacy and safety results. Journal of Hepatology 2011;54(Suppl 1):S482.
-
- Lawitz E, Gaultier I, Poordad F, DeJesus E, Kowdley KV, Sepulveda G, et al. 4‐week virologic response and safety of ABT‐450 given with low‐dose ritonavir (ABT‐450/r) in combination with pegylated interferon alpha‐2a and ribavirin (SOC) after 3‐day monotherapy in genotype 1 (GT1) HCV infected treatment‐naive subjects. Hepatology 2010;52(Suppl S1):878A.
Lawitz 2011b {published data only}
-
- Lawitz E, Jacobson I, Godofsky E, Foster GR, Flisiak R, Bennett M, et al. A phase 2b trial comparing 24 to 48 weeks treatment with tegobuvir (GS‐9190)/PEG/RBV to 48 weeks treatment with PEG/RBV for chronic genotype 1 HCV infection. Journal of Hepatology 2011;54(Suppl S1):S181.
Lawitz 2012a {published data only}
-
- Lawitz EJ, Gruener D, Hill JM, Marbury T, Moorehead L, Mathias A, et al. A phase 1, randomized, placebo‐controlled, 3‐day, dose‐ranging study of GS‐5885, an NS5A inhibitor, in patients with genotype 1 hepatitis C. Journal of Hepatology 2012;57(1):24‐31. - PubMed
Lawitz 2012b {published data only}
-
- Lawitz E, Hill J, Marbury T, Hazan L, Gruener D, Webster L, et al. GS‐6620, a liver‐targeted nucleotide prodrug, exhibits antiviral activity and favorable safety profile over 5 days in treatment naive chronic HCV genotype 1 subjects. Journal of Hepatology 2012;56(Suppl S2):S470‐1.
Lawitz 2013a1 {published data only}
-
- Lawitz E, Lalezari JP, Hassanein T, Kowdley KV, Poordad FF, Sheikh AM, et al. Sofosbuvir in combination with peginterferon alfa‐2a and ribavirin for non‐cirrhotic, treatment‐naive patients with genotypes 1, 2, and 3 hepatitis C infection: a randomised, double‐blind, phase 2 trial. Lancet Infectious Diseases 2013;13(5):401‐8. - PubMed
Lawitz 2013a2 {published data only}
-
- Lawitz E, Lalezari JP, Hassanein T, Kowdley KV, Poordad FF, Sheikh AM, et al. Sofosbuvir in combination with peginterferon alfa‐2a and ribavirin for non‐cirrhotic, treatment‐naive patients with genotypes 1, 2, and 3 hepatitis C infection: a randomised, double‐blind, phase 2 trial. Lancet Infectious Diseases 2013;13(5):401‐8. - PubMed
Lawitz 2013b {published data only}
-
- Lawitz E, Hill JM, Marbury TC, Rodriguez‐Torres M, DeMicco MP, Quesada J, et al. Three‐day, dose‐ranging study of the HCV NS3 protease inhibitor GS‐9451. Hepatology 2010;52(Suppl S1):714A.
-
- Lawitz EJ, Hill JM, Marbury T, Demicco MP, Delaney W, Yang J, et al. A phase I, randomized, placebo‐controlled, 3‐day, ascending‐dose study of GS‐9451, an NS3/4a protease inhibitor, in genotype 1 hepatitis C patients. Antiviral Therapy 2013;18(3):311‐9. - PubMed
Lawitz 2013c {published data only}
-
- Barnard R, Hwang PMT, Bhanja S, Campbell H, Strizki J, Cheney C, et al. Resistance analysis of cirrhotic treatment‐experienced genotype 1 patients in a study of MK‐7009 in combination with pegylated interferon/ribavirin. Journal of Hepatology 2013;58(Suppl S1):S487.
-
- Elbasha E, Khoury A, Mobashery N. Projected long‐term impact of MK‐7009 (vaniprevir) for previously treated chronic HCV genotype 1 infection. Hepatology International 2013;7(1 Suppl):S354.
-
- Gao W, Caro L, Anderson M, Hwang P, Zhou A, Su J, et al. Vaniprevir (MK‐7009) demonstrates higher exposures in treatment‐experienced genotype (GT) 1 cirrhotic than non‐cirrhotic HCV‐infected patients. Hepatology 2013;58(Suppl S1):739A.
-
- Lawitz E, Rodriguez‐Torres M, Stoehr A, Gane EJ, Serfaty L, Bhanja S, et al. A phase 2B study of MK‐7009 (vaniprevir) in patients with genotype 1 HCV infection who have failed previous pegylated interferon and ribavirin treatment. Journal of Hepatology 2013;59(1):11‐7. - PubMed
-
- Mobashery N, Wong P, Zhang S, Bhanja S, Warner A, Shaw PM, et al. Impact of IL28B genotype on virologic response in prior treatment failure patients who received MK‐7009 in combination with peginterferon and ribavirin. Journal of Hepatology 2012;56(Suppl S2):S467.
Lawitz 2013d {published data only}
-
- Lawitz E, Rodriguez‐Torres M, Denning JM, Albanis E, Cornpropst M, Berrey MM, et al. Pharmacokinetics, pharmacodynamics, and tolerability of GS‐9851, a nucleotide analog polymerase inhibitor, following multiple ascending doses in patients with chronic hepatitis C infection. Antimicrobial Agents and Chemotherapy 2013;57(3):1209‐17. - PMC - PubMed
Lawitz 2013e {published data only}
-
- Lawitz E, Sulkowski M, Jacobson IRA, Kraft Walter K, Maliakkal B, Al‐Ibrahim M, et al. Characterization of vaniprevir, a hepatitis C virus NS3/4A protease inhibitor, in patients with HCV genotype 1 infection: safety, antiviral activity, resistance, and pharmacokinetics. Antiviral Research 2013;99(3):214‐20. - PubMed
Lawitz 2013f {published data only}
-
- Lawitz E, Hill J, Vince B, Murillo A, Gruener D, Marbury T, et al. ACH‐2684 demonstrates potent viral suppression in genotype 1 hepatitis C patients with and without cirrhosis: safety, pharmacokinetic, and viral kinetic analysis. Journal of Hepatology 2013;58(Suppl S1):S347.
Lawitz 2014a {published data only}
-
- Lawitz E, Poordad F, Hyland RH, Wang J, Pang PS, Symonds WT, et al. High rates of SVR in patients with genotype 1 HCV infection and cirrhosis after treatment with ledipasvir/sofosbuvir+ribavirin or ledipasvir/sofosbuvir+ GS‐9669 for 8 weeks. Hepatology 2014;59(Suppl S1):1143A.
Lawitz 2015 {published data only}
-
- Lawitz E, Freilich B, Link J, German P, Mo H, Han L, et al. A phase 1, randomized, dose‐ranging study of GS‐5816, a once‐daily NS5A inhibitor, in patients with genotype 1‐4 hepatitis C virus. Journal of Viral Hepatitis 2015;22(12):1011‐9. [PUBMED: 26183611] - PubMed
-
- Lawitz E, Glass SJ, Gruener D, Freilich B. Hill JM, Link JO, et al. GS‐5816, a once‐daily NS5A inhibitor, demonstrates potent antiviral activity in patients with genotype 1, 2, 3, or 4 HCV infection in a 3‐day monotherapy study. Hepatology 2013;58(Suppl S1):731A.
Liu 2015a {published data only}
-
- Liu R, Curry S, McMonagle P, Nachbar RB, Pak I, Jumes P, et al. Resistance analysis of genotype‐1 and ‐3 HCV‐infected patients receiving MK‐8742, a HCV NS5A inhibitor with potent antiviral activity. Hepatology 2013;58(Suppl S1):435A.
-
- Yeh WW, Lipardi C, Jumes P, Lepeleire IM, Bulk N, Caro L, et al. MK‐8742, a HCV NS5A inhibitor with a broad spectrum of HCV genotypic activity, demonstrates potent antiviral activity in genotype‐1 and ‐3 HCV‐infected patients. Hepatology 2013;58(Suppl S1):438A‐9A.
Mallalieu 2014 {published data only}
-
- Lawitz E, Rodriguez‐Torres M, DeMicco M, Nguyen T, Godofsky E, Appleman J, et al. Antiviral activity of ANA598, a potent non‐nucleoside polymerase inhibitor, in chronic hepatitis C patients. Journal of Hepatology 2009;50(Suppl 1):S384.
-
- Mallalieu NL, Rahimy MH, Crowley CA, Appleman JR, Smith PF, Freddo JL. Pharmacokinetics and pharmacodynamics of setrobuvir, an orally administered hepatitis C virus non‐nucleoside analogue inhibitor. Clinical Therapeutics 2014;36(12):2047‐63. - PubMed
Manns 2011 {published data only}
-
- Manns MP, Bourliere M, Benhamou Y, Pol S, Bonacini M, Berg T. Safety and antiviral activity of BI201335, a new HCV NS3 protease inhibitor, in treatment‐naive patients with chronic hepatitis C genotype‐1 infection given as monotherapy and in combination with peginterferon alfa 2a (P) and ribavirin (R). Hepatology 2008;48(4 Suppl):1023A.
-
- Manns MP, Bourliere M, Benhamou Y, Pol S, Bonacini M, Trepo C, et al. Potency, safety, and pharmacokinetics of the NS3/4A protease inhibitor BI201335 in patients with chronic HCV genotype‐1 infection. Journal of Hepatology 2011;54(6):1114‐22. - PubMed
Manns 2012a1 {published data only}
-
- Lawitz E, Sulkowski M, Jacobson IRA, Kraft WK, Maliakkal B, Al‐Ibrahim M, et al. Characterization of vaniprevir, a hepatitis C virus NS3/4A protease inhibitor, in patients with HCV genotype 1 infection: safety, antiviral activity, resistance, and pharmacokinetics. Antiviral Research 2013;99(3):214‐20. - PubMed
-
- Manns MP, Gane E, Rodriguez‐Torres M, Stoehr A, Yeh C‐T, Marcellin P, et al. Vaniprevir with pegylated interferon alpha‐2a and ribavirin in treatment‐naive patients with chronic hepatitis C: a randomized phase II study. Hepatology 2012;56(3):884‐93. - PubMed
-
- Manns MP, Gane E, Rodriguez‐Torres M, Stoehr A, Yeh C‐T, Wiedmann R, et al. MK‐7009 significantly improves rapid viral response (RVR) in combination with pegylated interferon ALFA‐2A and ribavirin in patients with chronic hepatitis C (CHC) genotype 1 infection. Journal of Hepatology 2009;50(Suppl S1):S384.
-
- Manns MP, Gane E, Rodriguez‐Torres M, Stoehr A, Yeh CT, Marcellin P, et al. Vaniprevir with pegylated interferon alpha‐2a and ribavirin in treatment‐naive patients with chronic hepatitis C: a randomized phase II study. Hepatology 2012;56(3):884‐93. - PubMed
-
- Manns MP, Gane EJ, Rodriguez‐Torres M, Stoehr AD, Yeh C, Marcellin P, et al. Early viral response (EVR) rates in treatment ‐ naive patients with chronic hepatitis C (CHC) genotype 1 infection treated with MK‐7009, a novel NS3/4a protease inhibitor, in combination with pegylated interferon alfa‐2a and ribavirin for 28 days. Hepatology 2009;50(4 Suppl):332A‐3A.
Manns 2012a2 {published data only}
-
- Lawitz E, Sulkowski M, Jacobson IRA, Kraft WK, Maliakkal B, Al‐Ibrahim M, et al. Characterization of vaniprevir, a hepatitis C virus NS3/4A protease inhibitor, in patients with HCV genotype 1 infection: safety, antiviral activity, resistance, and pharmacokinetics. Antiviral Research 2013;99(3):214‐20. - PubMed
-
- Manns MP, Gane E, Rodriguez‐Torres M, Stoehr A, Yeh C‐T, Marcellin P, et al. Vaniprevir with pegylated interferon alpha‐2a and ribavirin in treatment‐naive patients with chronic hepatitis C: a randomized phase II study. Hepatology 2012;56(3):884‐93. - PubMed
-
- Manns MP, Gane E, Rodriguez‐Torres M, Stoehr A, Yeh C‐T, Wiedmann R, et al. MK‐7009 significantly improves rapid viral response (RVR) in combination with pegylated interferon ALFA‐2A and ribavirin in patients with chronic hepatitis C (CHC) genotype 1 infection. Journal of Hepatology 2009;50(Suppl S1):S384.
-
- Manns MP, Gane E, Rodriguez‐Torres M, Stoehr A, Yeh CT, Marcellin P, et al. Vaniprevir with pegylated interferon alpha‐2a and ribavirin in treatment‐naive patients with chronic hepatitis C: a randomized phase II study. Hepatology 2012;56(3):884‐93. - PubMed
-
- Manns MP, Gane EJ, Rodriguez‐Torres M, Stoehr AD, Yeh C, Marcellin P, et al. Early viral response (EVR) rates in treatment ‐ naive patients with chronic hepatitis C (CHC) genotype 1 infection treated with MK‐7009, a novel NS3/4a protease inhibitor, in combination with pegylated interferon alfa‐2a and ribavirin for 28 days. Hepatology 2009;50(4 Suppl):332A‐3A.
Manns 2012a3 {published data only}
-
- Lawitz E, Sulkowski M, Jacobson IRA, Kraft WK, Maliakkal B, Al‐Ibrahim M, et al. Characterization of vaniprevir, a hepatitis C virus NS3/4A protease inhibitor, in patients with HCV genotype 1 infection: safety, antiviral activity, resistance, and pharmacokinetics. Antiviral Research 2013;99(3):214‐20. - PubMed
-
- Manns MP, Gane E, Rodriguez‐Torres M, Stoehr A, Yeh C‐T, Marcellin P, et al. Vaniprevir with pegylated interferon alpha‐2a and ribavirin in treatment‐naive patients with chronic hepatitis C: a randomized phase II study. Hepatology 2012;56(3):884‐93. - PubMed
-
- Manns MP, Gane E, Rodriguez‐Torres M, Stoehr A, Yeh C‐T, Wiedmann R, et al. MK‐7009 significantly improves rapid viral response (RVR) in combination with pegylated interferon ALFA‐2A and ribavirin in patients with chronic hepatitis C (CHC) genotype 1 infection. Journal of Hepatology 2009;50(Suppl S1):S384.
-
- Manns MP, Gane E, Rodriguez‐Torres M, Stoehr A, Yeh CT, Marcellin P, et al. Vaniprevir with pegylated interferon alpha‐2a and ribavirin in treatment‐naive patients with chronic hepatitis C: a randomized phase II study. Hepatology 2012;56(3):884‐93. - PubMed
-
- Manns MP, Gane EJ, Rodriguez‐Torres M, Stoehr AD, Yeh C, Marcellin P, et al. Early viral response (EVR) rates in treatment ‐ naive patients with chronic hepatitis C (CHC) genotype 1 infection treated with MK‐7009, a novel NS3/4a protease inhibitor, in combination with pegylated interferon alfa‐2a and ribavirin for 28 days. Hepatology 2009;50(4 Suppl):332A‐3A.
Manns 2012a4 {published data only}
-
- Lawitz E, Sulkowski M, Jacobson IRA, Kraft WK, Maliakkal B, Al‐Ibrahim M, et al. Characterization of vaniprevir, a hepatitis C virus NS3/4A protease inhibitor, in patients with HCV genotype 1 infection: safety, antiviral activity, resistance, and pharmacokinetics. Antiviral Research 2013;99(3):214‐20. - PubMed
-
- Manns MP, Gane E, Rodriguez‐Torres M, Stoehr A, Yeh C‐T, Marcellin P, et al. Vaniprevir with pegylated interferon alpha‐2a and ribavirin in treatment‐naive patients with chronic hepatitis C: a randomized phase II study. Hepatology 2012;56(3):884‐93. - PubMed
-
- Manns MP, Gane E, Rodriguez‐Torres M, Stoehr A, Yeh C‐T, Wiedmann R, et al. MK‐7009 significantly improves rapid viral response (RVR) in combination with pegylated interferon ALFA‐2A and ribavirin in patients with chronic hepatitis C (CHC) genotype 1 infection. Journal of Hepatology 2009;50(Suppl S1):S384.
-
- Manns MP, Gane E, Rodriguez‐Torres M, Stoehr A, Yeh CT, Marcellin P, et al. Vaniprevir with pegylated interferon alpha‐2a and ribavirin in treatment‐naive patients with chronic hepatitis C: a randomized phase II study. Hepatology 2012;56(3):884‐93. - PubMed
-
- Manns MP, Gane EJ, Rodriguez‐Torres M, Stoehr AD, Yeh C, Marcellin P, et al. Early viral response (EVR) rates in treatment ‐ naive patients with chronic hepatitis C (CHC) genotype 1 infection treated with MK‐7009, a novel NS3/4a protease inhibitor, in combination with pegylated interferon alfa‐2a and ribavirin for 28 days. Hepatology 2009;50(4 Suppl):332A‐3A.
Manns 2014a {published data only}
-
- Manns M, Marcellin P, Poordad F, Araujo ESA, Buti M, Horsmans Y, et al. Simeprevir with pegylated interferon alfa 2a or 2b plus ribavirin in treatment‐naive patients with chronic hepatitis C virus genotype 1 infection (QUEST‐2): a randomised, double‐blind, placebo‐controlled phase 3 trial. Lancet 2014;384(9941):414‐26. - PubMed
-
- Poordad F, Manns MP, Marcellin P, Araujo ESA, Buti M, Horsmans Y, et al. Simeprevir (TMC435) with peginterferon/ribavirin for treatment of chronic HCV genotype‐1 infection in treatment‐naive patients: results from QUEST‐2, a phase III trial. Gastroenterology 2013;144(5 Suppl 1):S151.
Marcellin 2013a {published data only}
-
- Marcellin P, Popa S, Berliba A, Boyer N, Streinu‐Cercel A, Tong M, et al. ALS‐2200, a novel once‐daily nucleotide HCV polymerase inhibitor, demonstrates potent antiviral activity in treatment naive GT1 chronic hepatitis C patients. Hepatology International 2013;7(1 Suppl):S342.
Marcellin 2013b {published data only}
-
- Marcellin P, Manns MP, Janczewska E, Muir AJ, Wu X, Trenkle JD, et al. 12 week response‐guided treatment with the NS5A inhibitor, GS‐5885, the NS3 protease inhibitor, GS‐9451, plus pegylated interferon/ribavirin in treatment naive genotype 1 hepatitis C infected patients. Journal of Hepatology 2013;58(Suppl S1):S355.
MATTERHORN 2015a1 {published data only}
-
- Feld JJ, Jacobson I, Jensen DM, Foster GR, Pol S, Tam E, et al. Randomized study of danoprevir/ritonavir‐based therapy for HCV genotype 1 patients with prior partial or null responses to peginterferon/ribavirin. Journal of Hepatology 2015;62(2):294‐302. - PubMed
-
- Song ZZ. Randomized study of danoprevir/ritonavir‐based therapy for HCV genotype 1 patients with prior partial or null responses to peginterferon/ribavirin. Journal of Hepatology 2015;63(3):769‐70. - PubMed
MATTERHORN 2015a2 {published data only}
-
- Feld JJ, Jacobson I, Jensen DM, Foster GR, Pol S, Tam E, et al. Randomized study of danoprevir/ritonavir‐based therapy for HCV genotype 1 patients with prior partial or null responses to peginterferon/ribavirin. Journal of Hepatology 2015;62(2):294‐302. - PubMed
-
- Song ZZ. Randomized study of danoprevir/ritonavir‐based therapy for HCV genotype 1 patients with prior partial or null responses to peginterferon/ribavirin. Journal of Hepatology 2015;63(3):769‐70. - PubMed
McHutchison 2009 {published data only}
-
- Everson GT, Gordon SC, Jacobson I, Kauffman RS, McNair L, Muir A, et al. PROVE 1: subgroup analysis of a phase 2 study of telaprevir with peginterferon alfa‐2a and ribavirin in treatment‐naive subjects with hepatitis C. Hepatology International 2009;3:167.
-
- McHutchison JG, Everson GT, Gordon SC, Jacobson I, Kauffman R, McNair L, et al. PROVE1: results from a phase 2 study of telaprevir with peginterferon alfa‐2a and ribavirin in treatment‐naive subjects with hepatitis C. Journal of Hepatology 2008;48(Suppl S2):S4.
-
- McHutchison JG, Everson GT, Gordon SC, Jacobson IM, Sulkowski M, Kauffman R, et al. Telaprevir with peginterferon and ribavirin for chronic HCV genotype 1 infection. New England Journal of Medicine 2009;360(18):1827‐38. - PubMed
McHutchison 2010 {published data only}
-
- Everson GT, Gordon SC, Jacobson I, Kauffman RS, McNair L, Muir A, et al. PROVE 1: subgroup analysis of a phase 2 study of telaprevir with peginterferon alfa‐2a and ribavirin in treatment‐naive subjects with hepatitis C. Hepatology International 2009;3:167.
-
- Manns M, Muir A, Adda N, Jacobson I, Afdhal N, Heathcote J, et al. Telaprevir in hepatitis C genotype‐1‐infected patients with prior non‐response, viral breakthrough or relapse to peginterferonalfa‐2A/B and ribavirin therapy: SVR results of the PROVE3 study. Journal of Hepatology 2009;50(Suppl 1):S379.
-
- McHutchinson JG, Manns MP, Muir AJ, Terrault NA, Jacobson IM, Afdhal NH, et al. Telaprevir for previously treated chronic HCV infection. New England Journal of Medicine 2010;362(14):1292‐303. - PubMed
-
- McHutchinson JG, Shiffman ML, Terrault N, Manns MP, Biscegli AM, Jacobson IM, et al. Interim results of PROVE 3: a randomized, controlled, phase 2b study of telaprevir (TVR) with peginterferon‐alfa‐2a (P) and/or ribavirin (R) in subjects with hepatitis C genotype 1 who failed to achieve an SVR with a prior course of PR therapy. Hepatology International 2009;3(1):25.
-
- McHutchison JG, Manns MP, Muir A, Terrault N, Jacobson IM, Afdhal NH, et al. PROVE3 final results and 1‐year durability of SVR with telaprevir‐based regimen in hepatitis C genotype 1‐infected patients with prior non‐response, viral breakthrough or relapse to peginterferon‐alfa‐2a/b and ribavirin therapy. Hepatology 2009;50(4 Suppl):334A‐5A.
Mostafa 2015 {published data only}
-
- Mostafa I, Hassan M, Ibrahim AM, Khalafalla O, Essawy F, Abdelazem A, et al. Pilot study to determine the efficacy and safety of combining boceprevir with peginterferon alfa 2B and ribavirin in treatment naive patients with genotype 4 chronic hepatitis C infection. Hepatology International 2015;9(1 Suppl):S259.
Muir 2014 {published data only}
-
- Muir A, Poordad F, Sheikh A, Elkashab M, Brennan R, Ankoma‐Sey V, et al. SVR4 results for the combination of ACH‐3102 and Sovaprevir, with ribavirin, in patients with genotype 1 chronic hepatitis C infection. 23rd Annual Conference of APASL, Brisbane, Australia. 2014.
Nelson 2011 {published data only}
-
- Lawitz E, Lalezari JP, Hassanein T, Kowdley KV. Once daily PSI‐7977 plus PEG/RBV in treatment naive patients with HCV GT1: robust end of treatment response rates are sustained posttreatment. Hepatology 2011;54(Suppl S1):472A.
-
- Nelson DR, Lalezari J, Lawitz E, Hassanein T, Kowdley K, Poordad F, et al. Once daily PSI‐7977 plus PEG‐IFN/RBV in HCV GT1: 98% rapid virologic response, complete early virologic response: the proton study. Journal of Hepatology 2011;54(Suppl 1):S544.
Nelson 2012a1 {published data only}
Nelson 2012a2 {published data only}
Nelson 2012a3 {published data only}
Nelson 2012a4 {published data only}
Nelson 2012a5 {published data only}
Nelson 2012a6 {published data only}
Nelson 2012b {published data only}
-
- Nelson DR, Lawitz E, Bain V, Gitlin N, Hawkins T, Marotta P, et al. High SVR12 with 16 weeks of tegobuvir and GS‐9256 with peginterferon‐alfa 2A and ribavirin in treatment‐naïve genotype 1 HCV patients. Journal of Hepatology 2012;56(Suppl 2):S6‐7.
Nettles 2010 {published data only}
-
- Nettles RE, Chien C, Chung E, Persson A, Gao M, Belema M, et al. MS‐790052 is a first‐in class potent Hepatitis C Virus (HCV) NS5A inhibitor for patients with chronic HCV infection: results from a proof‐of‐concept study. Hepatology International 2010;4(1):184.
Nettles 2011a1 {published data only}
-
- Nettles R, Sevinsky H, Chung E, Burt D, Xiao H, Marbury TC, et al. BMS‐790052, a first‐in class potent hepatitis C virus NS5A inhibitor, demonstrates multiple dose proof‐of‐concept in subjects with chronic GT1 HCV infection. Hepatology 2010;52(Suppl S1):1214A‐5A.
-
- Nettles RE, Gao M, Bifano M, Chung E, Persson A, Marbury TC, et al. Multiple ascending dose study of BMS‐790052, a nonstructural protein 5A replication complex inhibitor, in patients infected with hepatitis C virus genotype 1. Hepatology 2011;54(6):1956‐65. - PubMed
Nettles 2011a2 {published data only}
-
- Nettles R, Sevinsky H, Chung E, Burt D, Xiao H, Marbury TC, et al. BMS‐790052, a first‐in class potent hepatitis C virus NS5A inhibitor, demonstrates multiple dose proof‐of‐concept in subjects with chronic GT1 HCV infection. Hepatology 2010;52(Suppl S1):1214A‐5A.
-
- Nettles RE, Gao M, Bifano M, Chung E, Persson A, Marbury TC, et al. Multiple ascending dose study of BMS‐790052, a nonstructural protein 5A replication complex inhibitor, in patients infected with hepatitis C virus genotype 1. Hepatology 2011;54(6):1956‐65. - PubMed
Nettles 2011a3 {published data only}
-
- Nettles R, Sevinsky H, Chung E, Burt D, Xiao H, Marbury TC, et al. BMS‐790052, a first‐in class potent hepatitis C virus NS5A inhibitor, demonstrates multiple dose proof‐of‐concept in subjects with chronic GT1 HCV infection. Hepatology 2010;52(Suppl S1):1214A‐5A.
-
- Nettles RE, Gao M, Bifano M, Chung E, Persson A, Marbury TC, et al. Multiple ascending dose study of BMS‐790052, a nonstructural protein 5A replication complex inhibitor, in patients infected with hepatitis C virus genotype 1. Hepatology 2011;54(6):1956‐65. - PubMed
Nettles 2011a4 {published data only}
-
- Nettles R, Sevinsky H, Chung E, Burt D, Xiao H, Marbury TC, et al. BMS‐790052, a first‐in class potent hepatitis C virus NS5A inhibitor, demonstrates multiple dose proof‐of‐concept in subjects with chronic GT1 HCV infection. Hepatology 2010;52(Suppl S1):1214A‐5A.
-
- Nettles RE, Gao M, Bifano M, Chung E, Persson A, Marbury TC, et al. Multiple ascending dose study of BMS‐790052, a nonstructural protein 5A replication complex inhibitor, in patients infected with hepatitis C virus genotype 1. Hepatology 2011;54(6):1956‐65. - PubMed
Nettles 2011a5 {published data only}
-
- Nettles R, Sevinsky H, Chung E, Burt D, Xiao H, Marbury TC, et al. BMS‐790052, a first‐in class potent hepatitis C virus NS5A inhibitor, demonstrates multiple dose proof‐of‐concept in subjects with chronic GT1 HCV infection. Hepatology 2010;52(Suppl S1):1214A‐5A.
-
- Nettles RE, Gao M, Bifano M, Chung E, Persson A, Marbury TC, et al. Multiple ascending dose study of BMS‐790052, a nonstructural protein 5A replication complex inhibitor, in patients infected with hepatitis C virus genotype 1. Hepatology 2011;54(6):1956‐65. - PubMed
Nettles 2011a6 {published data only}
-
- Nettles R, Sevinsky H, Chung E, Burt D, Xiao H, Marbury TC, et al. BMS‐790052, a first‐in class potent hepatitis C virus NS5A inhibitor, demonstrates multiple dose proof‐of‐concept in subjects with chronic GT1 HCV infection. Hepatology 2010;52(Suppl S1):1214A‐5A.
-
- Nettles RE, Gao M, Bifano M, Chung E, Persson A, Marbury TC, et al. Multiple ascending dose study of BMS‐790052, a nonstructural protein 5A replication complex inhibitor, in patients infected with hepatitis C virus genotype 1. Hepatology 2011;54(6):1956‐65. - PubMed
Nishiguchi 2014a1 {published data only}
-
- Nishiguchi S, Kuboki M, Sakai Y, Aizawa N, Iwata K, Ikeda N, et al. All treated Japanese genotype 1 patients with IL28B TT allele achieved SVR by 4 weeks faldaprevir and 48 weeks PegIFN/ ribavirin. Hepatology International 2013;7(1 Suppl):S393.
-
- Nishiguchi S, Kuboki M, Sakai Y, Aizawa N, Takashima T, Iwata K. 100% SVR in Japanese genotype 1 patients with IL28B TT allele treated with 4 weeks faldaprevir and 48 weeks PegIFN/ribavirin. Hepatology International 2013;7(1 Suppl):S372‐3.
-
- Nishiguchi S, Sakai Y, Kuboki M, Tsunematsu S, Urano Y, Sakamoto W, et al. Safety and efficacy of faldaprevir with pegylated interferon alfa‐2a and ribavirin in Japanese patients with chronic genotype‐1 hepatitis C infection. Liver International 2014;34(1):78‐88. - PubMed
Nishiguchi 2014a2 {published data only}
-
- Nishiguchi S, Kuboki M, Sakai Y, Aizawa N, Iwata K, Ikeda N, et al. All treated Japanese genotype 1 patients with IL28B TT allele achieved SVR by 4 weeks faldaprevir and 48 weeks PegIFN/ ribavirin. Hepatology International 2013;7(1 Suppl):S393.
-
- Nishiguchi S, Kuboki M, Sakai Y, Aizawa N, Takashima T, Iwata K. 100% SVR in Japanese genotype 1 patients with IL28B TT allele treated with 4 weeks faldaprevir and 48 weeks PegIFN/ribavirin. Hepatology International 2013;7(1 Suppl):S372‐3.
-
- Nishiguchi S, Sakai Y, Kuboki M, Tsunematsu S, Urano Y, Sakamoto W, et al. Safety and efficacy of faldaprevir with pegylated interferon alfa‐2a and ribavirin in Japanese patients with chronic genotype‐1 hepatitis C infection. Liver International 2014;34(1):78‐88. - PubMed
OPERA 2011a1 {published data only}
-
- Manns M, Reesink H, Berg T, Dusheiko G, Flisiak R, Marcellin P, et al. Rapid viral response of once‐daily TMC435 plus pegylated interferon/ribavirin in hepatitis C genotype‐1 patients: a randomized trial. Antiviral Therapy 2011;16(7):1021‐33. - PubMed
OPERA 2011a2 {published data only}
-
- Manns M, Reesink H, Berg T, Dusheiko G, Flisiak R, Marcellin P, et al. Rapid viral response of once‐daily TMC435 plus pegylated interferon/ribavirin in hepatitis C genotype‐1 patients: a randomized trial. Antiviral Therapy 2011;16(7):1021‐33. - PubMed
OPERA 2011a3 {published data only}
-
- Manns M, Reesink H, Berg T, Dusheiko G, Flisiak R, Marcellin P, et al. Rapid viral response of once‐daily TMC435 plus pegylated interferon/ribavirin in hepatitis C genotype‐1 patients: a randomized trial. Antiviral Therapy 2011;16(7):1021‐33. - PubMed
OPERA 2011a4 {published data only}
-
- Manns M, Reesink H, Berg T, Dusheiko G, Flisiak R, Marcellin P, et al. Rapid viral response of once‐daily TMC435 plus pegylated interferon/ribavirin in hepatitis C genotype‐1 patients: a randomized trial. Antiviral Therapy 2011;16(7):1021‐33. - PubMed
OPERA 2011a5 {published data only}
-
- Manns M, Reesink H, Berg T, Dusheiko G, Flisiak R, Marcellin P, et al. Rapid viral response of once‐daily TMC435 plus pegylated interferon/ribavirin in hepatitis C genotype‐1 patients: a randomized trial. Antiviral Therapy 2011;16(7):1021‐33. - PubMed
OPERA 2011a6 {published data only}
-
- Manns M, Reesink H, Berg T, Dusheiko G, Flisiak R, Marcellin P, et al. Rapid viral response of once‐daily TMC435 plus pegylated interferon/ribavirin in hepatitis C genotype‐1 patients: a randomized trial. Antiviral Therapy 2011;16(7):1021‐33. - PubMed
Pasquinelli 2012a1 {published data only}
-
- Pasquinelli C, Eley T, Villegas C, Sandy K, Mathias E, Wendelburg P, et al. Safety, tolerability, pharmacokinetics and antiviral activity following single and multiple‐dose administration of BMS‐650032, a novel HCV NS3 inhibitor, in subjects with chronic genotype 1 HCV infection. Hepatology International 2010;4(1):184.
-
- Pasquinelli C, Eley T, Villegas C, Sandy K, Mathias E, Wendelburg P, et al. Safety, tolerability, pharmacokinetics and antiviral activity following single‐and multiple‐dose administration of BMS‐650032, a novel HCV NS3 inhibitor, in subjects with chronic genotype 1 HCV infection. Hepatology 2009;50(4 Suppl):411A‐2A.
Pasquinelli 2012a2 {published data only}
-
- Pasquinelli C, Eley T, Villegas C, Sandy K, Mathias E, Wendelburg P, et al. Safety, tolerability, pharmacokinetics and antiviral activity following single and multiple‐dose administration of BMS‐650032, a novel HCV NS3 inhibitor, in subjects with chronic genotype 1 HCV infection. Hepatology International 2010;4(1):184.
-
- Pasquinelli C, Eley T, Villegas C, Sandy K, Mathias E, Wendelburg P, et al. Safety, tolerability, pharmacokinetics and antiviral activity following single‐and multiple‐dose administration of BMS‐650032, a novel HCV NS3 inhibitor, in subjects with chronic genotype 1 HCV infection. Hepatology 2009;50(4 Suppl):411A‐2A.
Pearlman 2014 {published data only}
-
- Pearlman BL, Ehleben C. Hepatitis C genotype 1 virus with low viral load and rapid virologic response to peginterferon/ribavirin obviates a protease inhibitor. Hepatology 2014;59(1):71‐7. - PubMed
Pearlman 2015 {published data only}
-
- Pearlman BL, Ehleben C, Perrys M. The combination of simeprevir and sofosbuvir is more effective than that of peginterferon, ribavirin, and sofosbuvir for patients with hepatitis C‐related Child's class A cirrhosis. Gastroenterology 2015;148(4):762. - PubMed
Petry 2011 {published data only}
-
- Petry AS, Fraser IP, OMara E, Dyck K, Nachbar RB, Lepeleire IM, et al. Safety and antiviral activity of MK‐5172, a next generation HCV NS3/4A protease inhibitor with a broad HCV genotypic activity spectrum and potent activity against known resistance mutants, in genotype 1 and 3 HCV infected patients. Hepatology 2011;54(4 Suppl):531A.
Pockros 2008a1 {published data only}
-
- Nelson D, Pockros PJ, Godofsky E, Rodriguez‐Torres M, Everson G, Fried M, et al. High end‐of‐treatment response (84%) after 4 weeks of R1626, peginterferon alfa‐2A (40KD) and ribavirin followed by a further 44 weeks of peginterferon alfa‐2A and ribavirin. Journal of Hepatology 2008;48(Suppl 2):S371.
-
- Pockros PJ, Nelson D, Godofsky E, Rodriguez‐Torres M, Everson GT, Fried MW, et al. R1626 plus peginterferon alfa‐2a provides potent suppression of hepatitis C virus RNA and significant antiviral synergy in combination with ribavirin. Hepatology 2008;48(2):385‐97. - PubMed
Pockros 2008a2 {published data only}
-
- Nelson D, Pockros PJ, Godofsky E, Rodriguez‐Torres M, Everson G, Fried M, et al. High end‐of‐treatment response (84%) after 4 weeks of R1626, peginterferon alfa‐2A (40KD) and ribavirin followed by a further 44 weeks of peginterferon alfa‐2A and ribavirin. Journal of Hepatology 2008;48(Suppl 2):S371.
-
- Pockros PJ, Nelson D, Godofsky E, Rodriguez‐Torres M, Everson GT, Fried MW, et al. R1626 plus peginterferon alfa‐2a provides potent suppression of hepatitis C virus RNA and significant antiviral synergy in combination with ribavirin. Hepatology 2008;48(2):385‐97. - PubMed
Pockros 2008a3 {published data only}
-
- Nelson D, Pockros PJ, Godofsky E, Rodriguez‐Torres M, Everson G, Fried M, et al. High end‐of‐treatment response (84%) after 4 weeks of R1626, peginterferon alfa‐2A (40KD) and ribavirin followed by a further 44 weeks of peginterferon alfa‐2A and ribavirin. Journal of Hepatology 2008;48(Suppl 2):S371.
-
- Pockros PJ, Nelson D, Godofsky E, Rodriguez‐Torres M, Everson GT, Fried MW, et al. R1626 plus peginterferon alfa‐2a provides potent suppression of hepatitis C virus RNA and significant antiviral synergy in combination with ribavirin. Hepatology 2008;48(2):385‐97. - PubMed
Pockros 2009 {published data only}
-
- Pockros P, Rodriguez‐Torres M, Villano S, Maller E, Chojkier M. A phase 2, randomized study of HCV‐796 in combination with pegylated‐interferon (PEG) plus ribavirin (RBV) versus peg plus RBV in hepatitis C virus genotype‐1 infection. Journal of Hepatology 2009;50(Suppl S1):S7.
Pol 2012 {published data only}
-
- Pol S, Ghalib RH, Rustgi VK, Martorell C, Everson GT, Tatum HA, et al. Daclatasvir for previously untreated chronic hepatitis C genotype‐1 infection: a randomised, parallel‐group, double‐blind, placebo‐controlled, dose‐finding, phase 2a trial. Lancet Infectious Diseases 2012;12(9):671‐7. - PubMed
Pol 2013 {published data only}
-
- Pol S, Jablkowski M, Trenkle JD, Kanwar B, Bekele BN, Subramanian M, et al. Antiviral efficacy of the NS3 protease inhibitor, GS‐9451, non‐nucleoside NS5b inhibitor, tegobuvir, and pegylated interferon plus ribavirin in treatment‐naive genotype 1 hepatitis C infected patients. Journal of Hepatology 2013;58(Suppl S1):S31.
Poordad 2007 {published data only}
-
- Poordad F, Lawitz EJ, Gitlin N, Rodriguez‐Torres M, Box T, Nguyen T, et al. Efficacy and safety of valopicitabine in combination with pegylated interferon (Peg IFN) and ribavirin (RBV) in patients with chronic hepatitis C. Hepatology 2007;46(4 Suppl 1):866A.
Poordad 2011a1 {published data only}
-
- Albrecht J, Vierling J, Kwo P, Lawitz E, McCone J, Schiff E. Frequencies of resistance‐associated amino acid variants (RAV) following treatment with boceprevir (BOC) plus peginterferon alfa‐2b and ribavirin (P/R). Hepatology International 2011;5(1):261‐2.
-
- Barnard RJO, Pedicone LD, Chaudhri E, Tong X, Qiu P, Brass CA. Frequencies of resistance‐associated amino acid variants following combination treatment with boceprevir (BOC) plus Pegintron (peglnterferon alfa‐2b) and ribavirin (P/R) in patients with chronic hepatitis C (CHC), genotype 1 (G1). Journal of the International Association of Physicians in AIDS Care 2011;10(3):199‐200.
-
- Bronowicki J, McCone J, Bacon BR, Bruno S, Manns MP, Sulkowski MS, et al. Response‐guided therapy (RGT) with boceprevir (BOC) + peginterferon alfa‐2b/ribavirin (P/R) for treatment‐naive patients with hepatitis C virus (HCV) genotype (G) 1 was similar to a 48‐wk fixed‐duration regimen with BOC + P/R in SPRINT‐2. Hepatology 2010;52(Suppl S1):881A.
-
- Charlton M. Boceprevir (Victrelis) for HCV: V is for victory and very complex. Hepatology 2011;54(5):1882‐6. - PubMed
-
- Manns MP, Markova AA, Serrano BC, Cornberg M. Phase III results of boceprevir in treatment naive patients with chronic hepatitis C genotype 1. Liver International 2012;32(Suppl 1):27‐31. - PubMed
Poordad 2011a2 {published data only}
-
- Bronowicki J, McCone J, Bacon BR, Bruno S, Manns MP, Sulkowski MS, et al. Response‐guided therapy (RGT) with boceprevir (BOC) + peginterferon alfa‐2b/ribavirin (P/R) for treatment‐naive patients with hepatitis C virus (HCV) genotype (G) 1 was similar to a 48‐wk fixed‐duration regimen with BOC + P/R in SPRINT‐2. Hepatology 2010;52(Suppl S1):881A.
-
- Charlton M. Boceprevir (Victrelis) for HCV: V is for victory and very complex. Hepatology 2011;54(5):1882‐6. - PubMed
-
- Manns MP, Markova AA, Serrano BC, Cornberg M. Phase III results of boceprevir in treatment naive patients with chronic hepatitis C genotype 1. Liver International 2012;32(Suppl 1):27‐31. - PubMed
-
- Manns MP, McCone J, Davis M, Shiffman ML, Rossaro L, Bourliere M, et al. Safety benefits of response‐guided therapy with boceprevir (BOC) plus peginterferon alfa‐2B/ribavirin (PR) in previously untreated patients with HCV genotype 1 infection. Hepatology 2011;54(4 Suppl):813A.
-
- McCone J, Jacobson IM, Bacon BR, Pedicone L, Goteti VS, Burroughs M, et al. Treatment‐naive black patients treated with boceprevir combined with peginterferon alfa‐2b + ribavirin: results from HCV sprint‐2. Hepatology 2011;54(4 Suppl):822A‐3A.
POSITRON 2013 {published data only}
-
- Jacobson IM, Gordon SC, Kowdley KV, Yoshida EM, Rodriguez‐Torres M, Sulkowski MS, et al. Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment options. New England Journal of Medicine 2013;368(20):1867‐77. - PubMed
-
- Younossi ZM, Stepanova M, Henry L, Gane E, Jacobson IM, Lawitz E, et al. Minimal impact of sofosbuvir and ribavirin on health related quality of life in chronic hepatitis C (CH‐C). Journal of Hepatology 2014;60(4):741‐7. [PUBMED: 24333184] - PubMed
Reddy 2007 {published data only}
-
- Reddy R, Rodriguez‐Torres M, Gane E, Robson R, Lalezari J, Everson GT, et al. Antiviral activity, pharmacokinetics, safety, and tolerability of R7128, a novel nucleoside HCV RNA polymerase inhibitor, following multiple, ascending, oral doses in patients with HCV genotype 1 infection who have failed prior interferon therapy. Hepatology 2007;46(Suppl S1):862A‐3A.
Reesink 2006 {published data only}
-
- Reesink HW, Zeuzem S, Vliet A, McNair L, Purdy S, Chu H, et al. Initial results of a phase 1b, multiple‐dose study of VX‐950, a hepatitis C virus protease inhibitor. Gastroenterology 2005;128(4 Suppl 2):A697.
-
- Reesink HW, Zeuzem S, Weegink CJ, Forestier N, Vliet A, Wetering De Rooij J, et al. Rapid decline of viral RNA in hepatitis C patients treated with VX‐950: a phase Ib, placebo‐controlled, randomized study. Gastroenterology 2006;131(4):997‐1002. - PubMed
Reiser 2005 {published data only}
-
- Reiser M, Hinrichsen H, Benhamou Y, Reesink HW, Wedemeyer H, Avendano C, et al. Antiviral efficacy of NS3‐serine protease inhibitor BILN‐2061 in patients with chronic genotype 2 and 3 hepatitis C. Hepatology (Baltimore, Md.) 2005;41(4):832‐5. - PubMed
Rodriguez‐Torres 2008 {published data only}
-
- Rodriguez‐Torres M, Lalezari J, Gane EJ, DeJesus E, Nelson DR, Everson GT, et al. Potent antiviral response to the HCV nucleoside polymerase inhibitor R7128 for 28 days with peg‐ifn and ribavirin: subanalysis by race/ethnicity, weight and HCV genotype. Hepatology 2008;48(4 Suppl):1160A.
Rodriguez‐Torres 2010 {published data only}
-
- Rodriguez‐Torres M, Lawitz E, Conway B, Kaita K, Sheikh AM, Ghalib R, et al. Safety and antiviral activity of the HCV non‐nucleoside polymerase inhibitor VX‐222 in treatment‐naive genotype 1 HCV infected patients. Journal of Hepatology 2010;52(Suppl S1):S14.
Rodriguez‐Torres 2011a1 {published data only}
-
- Rodriguez‐Torres M, Lawitz E, Hazan L, Barry AN, Wenzel ED, Alam J. Antiviral activity and safety of INX‐08189, a nucleotide polymerase inhibitor, following 7‐days of oral therapy in naive genotype‐1 chronic HCV patients. Hepatology 2011;54(Suppl S1):535A.
Rodriguez‐Torres 2011a2 {published data only}
-
- Rodriguez‐Torres M, Lawitz E, Denning J, Cornpropst M, Albanis E, Symonds W, et al. PSI‐352938, a novel purine nucleotide analog, exhibits potent antiviral activity and no evidence of resistance in patients with HCV genotype 1 over 7 days. Journal of Hepatology 2011;54(Suppl S1):S488.
Rodriguez‐Torres 2013 {published data only}
-
- Rodriguez‐Torres M, Lawitz E, Kowdley KV, Nelson DR, Dejesus E, McHutchison JG, et al. Sofosbuvir (GS‐7977) plus peginterferon/ribavirin in treatment‐naïve patients with HCV genotype 1: a randomized, 28‐day, dose‐ranging trial. Journal of Hepatology 2013;58(4):663‐8. - PubMed
Rodriguez‐Torres 2014a1 {published data only}
-
- Rodriguez‐Torres M, Stoehr A, Gane EJ, Serfaty L, Lawitz E, Zhou A, et al. Combination of vaniprevir with peginterferon and ribavirin significantly increases the rate of SVR in treatment‐experienced patients with chronic HCV genotype 1 infection and cirrhosis. Clinical Gastroenterology and Hepatology 2014;12(6):1029‐37.e5. - PubMed
Rodriguez‐Torres 2014a2 {published data only}
-
- Rodriguez‐Torres M, Stoehr A, Gane EJ, Serfaty L, Lawitz E, Zhou A, et al. Combination of vaniprevir with peginterferon and ribavirin significantly increases the rate of SVR in treatment‐experienced patients with chronic HCV genotype 1 infection and cirrhosis. Clinical Gastroenterology and Hepatology 2014;12(6):1029‐37.e5. - PubMed
Rodriguez‐Torres 2014a3 {published data only}
-
- Rodriguez‐Torres M, Stoehr A, Gane EJ, Serfaty L, Lawitz E, Zhou A, et al. Combination of vaniprevir with peginterferon and ribavirin significantly increases the rate of SVR in treatment‐experienced patients with chronic HCV genotype 1 infection and cirrhosis. Clinical Gastroenterology and Hepatology 2014;12(6):1029‐37.e5. - PubMed
Rodriguez‐Torres 2014a4 {published data only}
-
- Rodriguez‐Torres M, Stoehr A, Gane EJ, Serfaty L, Lawitz E, Zhou A, et al. Combination of vaniprevir with peginterferon and ribavirin significantly increases the rate of SVR in treatment‐experienced patients with chronic HCV genotype 1 infection and cirrhosis. Clinical Gastroenterology and Hepatology 2014;12(6):1029‐37.e5. - PubMed
Rodriguez‐Torres 2014b1 {published data only}
-
- Rodriguez‐Torres M, Yoshida EM, Marcellin P, Srinivasan S, Purohit VS, Wang C, et al. A phase 2 study of filibuvir in combination with pegylated IFN alfa and ribavirin for chronic HCV. Annals of Hepatology 2014;13(4):364‐75. - PubMed
Rodriguez‐Torres 2014b2 {published data only}
-
- Rodriguez‐Torres M, Yoshida EM, Marcellin P, Srinivasan S, Purohit VS, Wang C, et al. A phase 2 study of filibuvir in combination with pegylated IFN alfa and ribavirin for chronic HCV. Annals of Hepatology 2014;13(4):364‐75. - PubMed
Rodriguez‐Torres 2015 {published data only}
-
- Rodriguez‐Torres M, Glass S, Hill J, Freilich B, Hassman D, Bisceglie A, et al. The pan‐genotypic NS3/4A protease inhibitor GS‐9857 demonstrates potent antiviral activity in patients infected with HCV genotype 1, 2, 3 or 4 in a 3‐day monotherapy study. Journal of Hepatology 2015;62(Suppl S2):S682.
Sarrazin 2007 {published data only}
-
- Sarrazin C, Rouzier R, Wagner F, Forestier N, Larrey D, Gupta SK. SCH 503034, a novel hepatitis C virus protease inhibitor, plus pegylated interferon alpha‐2b for genotype 1 nonresponders. Gastroenterology 2007;132(4):1270‐8. - PubMed
-
- Vermehren J, Susser S, Karey U, Forestier N, Lange CM, Hughes EA, et al. Clonal analysis of mutations selected in the HCV NS3 protease domain of genotype 1 non‐responders sequentially treated with boceprevir (SCH503034) and/or pegylated interferon alfa‐2b (PEG‐IFN α‐2b). Hepatology 2009;50(4 Suppl):1040A‐1A.
Schiff 2008 {published data only}
-
- Schiff E, Poordad F, Jacobson I, Flamm S, Bacon B, Lawitz E, et al. Boceprevir (B) combination therapy in null responders (NR): response dependent on interferon responsiveness. Journal of Hepatology 2008;48(Suppl 2):S46.
Silva 2013a1 {published data only}
-
- Silva MO, Treitel M, Graham DJ, Curry S, Frontera MJ, McMonagle P, et al. Antiviral activity of boceprevir monotherapy in treatment‐naive subjects with chronic hepatitis C genotype 2/3. Journal of Hepatology 2013;59(1):31‐7. - PubMed
Silva 2013a2 {published data only}
-
- Silva MO, Treitel M, Graham DJ, Curry S, Frontera MJ, McMonagle P, et al. Antiviral activity of boceprevir monotherapy in treatment‐naive subjects with chronic hepatitis C genotype 2/3. Journal of Hepatology 2013;59(1):31‐7. - PubMed
Silva 2013a3 {published data only}
-
- Silva MO, Treitel M, Graham DJ, Curry S, Frontera MJ, McMonagle P, et al. Antiviral activity of boceprevir monotherapy in treatment‐naive subjects with chronic hepatitis C genotype 2/3. Journal of Hepatology 2013;59(1):31‐7. - PubMed
Sims 2014 {published data only}
STARTVerso‐1 2015a1 {published data only}
-
- Asselah T, Jensen DM, Foster G, Sulkowski M, Ouzan D, Morano L, et al. Faldaprevir plus pegylated interferon/ribavirin did not increase anaemia compared with pegylated interferon/ribavirin in HCV genotype‐1, treatment‐naive patients: pooled analysis of phase III studies. Gastroenterology 2014;146(5 Suppl 1):S‐977.
-
- Asselah T, Jensen DM, Foster G, Sulkowski M, Ouzan D, Morano L, et al. Virological response in treatment‐naive patients with chronic HCV genotype‐1 infection receiving faldaprevir plus pegylated interferon alpha‐2a and ribavirin is unaffected by ribavirin dose reduction. Gastroenterology 2014;146(5 Suppl 1):S975‐6.
-
- Berger K, Sarrazin C, Ferenci P, Jensen DM, Jacobson IM, Stern JO, et al. NS3 Q80K did not impact efficacy or treatment‐emergent resistance patterns in HCV genotype 1‐infected patients receiving faldaprevir + peginterferon/ribavirin in three phase III trials. Journal of Hepatology 2014;60(1 Suppl S):S497.
-
- Ferenci P, Asselah T, Foster GR, Zeuzem S, Sarrazin C, Moreno C, et al. Faldaprevir plus pegylated interferon alfa‐2A and ribavirin in chronic HCV genotype‐1 treatment‐naive patients: final results from STARTVerso1, a randomised, double‐blind, placebo‐controlled phase III trial. Journal of Gastroenterology and Hepatology 2013;28(Suppl 2):157‐8.
-
- Ferenci P, Asselah T, Foster GR, Zeuzem S, Sarrazin C, Moreno C, et al. STARTVerso1: a randomized trial of faldaprevir plus pegylated interferon/ribavirin for chronic HCV genotype‐1 infection. Journal of Hepatology 2015;62(6):1246‐55. - PubMed
STARTVerso‐1 2015a2 {published data only}
-
- Asselah T, Jensen DM, Foster G, Sulkowski M, Ouzan D, Morano L, et al. Faldaprevir plus pegylated interferon/ribavirin did not increase anaemia compared with pegylated interferon/ribavirin in HCV genotype‐1, treatment‐naive patients: pooled analysis of phase III studies. Gastroenterology 2014;146(5 Suppl 1):S‐977.
-
- Asselah T, Jensen DM, Foster G, Sulkowski M, Ouzan D, Morano L, et al. Virological response in treatment‐naive patients with chronic HCV genotype‐1 infection receiving faldaprevir plus pegylated interferon alpha‐2a and ribavirin is unaffected by ribavirin dose reduction. Gastroenterology 2014;146(5 Suppl 1):S975‐6.
-
- Berger K, Sarrazin C, Ferenci P, Jensen DM, Jacobson IM, Stern JO, et al. NS3 Q80K did not impact efficacy or treatment‐emergent resistance patterns in HCV genotype 1‐infected patients receiving faldaprevir + peginterferon/ribavirin in three phase III trials. Journal of Hepatology 2014;60(1 Suppl S):S497.
-
- Ferenci P, Asselah T, Foster GR, Zeuzem S, Sarrazin C, Moreno C, et al. Faldaprevir plus pegylated interferon alfa‐2A and ribavirin in chronic HCV genotype‐1 treatment‐naive patients: final results from STARTVerso1, a randomised, double‐blind, placebo‐controlled phase III trial. Journal of Gastroenterology and Hepatology 2013;28(Suppl 2):157‐8.
-
- Ferenci P, Asselah T, Foster GR, Zeuzem S, Sarrazin C, Moreno C, et al. STARTVerso1: a randomized trial of faldaprevir plus pegylated interferon/ribavirin for chronic HCV genotype‐1 infection. Journal of Hepatology 2015;62(6):1246‐55. - PubMed
STARTverso‐2 2014a1 {published data only}
-
- Asselah T, Jensen DM, Foster G, Sulkowski M, Ouzan D, Morano L, et al. Faldaprevir plus pegylated interferon/ribavirin did not increase anaemia compared with pegylated interferon/ribavirin in HCV genotype‐1, treatment‐naive patients: pooled analysis of phase III studies. Gastroenterology 2014;146(5 (Suppl 1)):S‐977.
-
- Asselah T, Jensen DM, Foster G, Sulkowski M, Ouzan D, Morano L, et al. Virological response in treatment‐naive patients with chronic HCV genotype‐1 infection receiving faldaprevir plus pegylated interferon alpha‐2a and ribavirin is unaffected by ribavirin dose reduction. Gastroenterology 2014;146(5 (Suppl 1)):S975‐6.
-
- Berger K, Sarrazin C, Ferenci P, Jensen DM, Jacobson IM, Stern JO, et al. NS3 Q80K did not impact efficacy or treatment‐emergent resistance patterns in HCV genotype 1‐infected patients receiving faldaprevir + peginterferon/ribavirin in three phase III trials. Journal of Hepatology 2014;60(1):S497.
-
- Ferenci P, Dieterich D, Zeuzem S, Mantry P, Sarrazin C, Crespo J, et al. Early stopping rules for faldaprevir plus pegylated interferon alpha‐2a and ribavirin in treatment‐naive patients: exploratory study of pooled data from phase III trials. Journal of Hepatology 2014;60(1 (Suppl 1)):S452‐3.
-
- Foster GR, Cooper C, Dieterich D, Ferenci P, Crespo J, Diago M, et al. Pharmacokinetic‐response analysis of faldaprevir in treatment‐naive patients with chronic HCV genotype‐1 infection: a pooled analysis of two phase III trials. Journal of Hepatology 2014;60(1 (Suppl 1)):S454‐5.
STARTverso‐2 2014a2 {published data only}
-
- Asselah T, Jensen DM, Foster G, Sulkowski M, Ouzan D, Morano L, et al. Faldaprevir plus pegylated interferon/ribavirin did not increase anaemia compared with pegylated interferon/ribavirin in HCV genotype‐1, treatment‐naive patients: pooled analysis of phase III studies. Gastroenterology 2014;146(5 (Suppl 1)):S‐977.
-
- Asselah T, Jensen DM, Foster G, Sulkowski M, Ouzan D, Morano L, et al. Virological response in treatment‐naive patients with chronic HCV genotype‐1 infection receiving faldaprevir plus pegylated interferon alpha‐2a and ribavirin is unaffected by ribavirin dose reduction. Gastroenterology 2014;146(5 (Suppl 1)):S975‐6.
-
- Berger K, Sarrazin C, Ferenci P, Jensen DM, Jacobson IM, Stern JO, et al. NS3 Q80K did not impact efficacy or treatment‐emergent resistance patterns in HCV genotype 1‐infected patients receiving faldaprevir + peginterferon/ribavirin in three phase III trials. Journal of Hepatology 2014;60(1):S497.
-
- Ferenci P, Dieterich D, Zeuzem S, Mantry P, Sarrazin C, Crespo J, et al. Early stopping rules for faldaprevir plus pegylated interferon alpha‐2a and ribavirin in treatment‐naive patients: exploratory study of pooled data from phase III trials. Journal of Hepatology 2014;60(1 (Suppl 1)):S452‐3.
-
- Foster GR, Cooper C, Dieterich D, Ferenci P, Crespo J, Diago M, et al. Pharmacokinetic‐response analysis of faldaprevir in treatment‐naive patients with chronic HCV genotype‐1 infection: a pooled analysis of two phase III trials. Journal of Hepatology 2014;60(1 (Suppl 1)):S454‐5.
STARTverso‐3 2013a1 {published data only}
-
- Asselah T, Zehnter E, Agarwal K, Sakai Y, Yatsuhashi H, Willems B, et al. Pharmacokinetic‐response analysis of faldaprevir in patients with chronic HCV genotype‐1 infection with prior relapse. Journal of Hepatology 2014;60(1 Suppl S):S458‐9.
-
- Berger K, Sarrazin C, Ferenci P, Jensen DM, Jacobson IM, Stern JO, et al. NS3 Q80K did not impact efficacy or treatment‐emergent resistance patterns in HCV genotype 1‐infected patients receiving faldaprevir+peginterferon/ribavirin in three phase III trials. Journal of Hepatology 2014;60(1 Suppl S):S497.
-
- Jacobson IM, Asselah T, Ferenci P, Foster GR, Jensen DM, Negro F, et al. STARTVerso3: a randomized, double‐blind, placebo‐controlled phase III trial of faldaprevir in combination with pegylated interferon a lf a‐2a and ribavirin in treatment‐experienced patients with chronic hepatitis C genotype‐1 infection. Hepatology 2013;48(S1):742A‐3A.
-
- Jacobson IM, Ferenci P, Foster GR, Jensen DM, Negro F, Mantry P, et al. STARTVerso3: a randomized, double‐blind, placebo controlled phase III trial of faldaprevir in combination with pegylated interferon alpha‐2a and ribavirin in treatment experienced patients with chronic hepatitis C genotype‐1 infection. Hepatology International 2014;8(1 Suppl):S213‐4.
STARTverso‐3 2013a2 {published data only}
-
- Asselah T, Zehnter E, Agarwal K, Sakai Y, Yatsuhashi H, Willems B, et al. Pharmacokinetic‐response analysis of faldaprevir in patients with chronic HCV genotype‐1 infection with prior relapse. Journal of Hepatology 2014;60(1 Suppl S):S458‐9.
-
- Berger K, Sarrazin C, Ferenci P, Jensen DM, Jacobson IM, Stern JO, et al. NS3 Q80K did not impact efficacy or treatment‐emergent resistance patterns in HCV genotype 1‐infected patients receiving faldaprevir+peginterferon/ribavirin in three phase III trials. Journal of Hepatology 2014;60(1 Suppl S):S497.
-
- Jacobson IM, Asselah T, Ferenci P, Foster GR, Jensen DM, Negro F, et al. STARTVerso3: a randomized, double‐blind, placebo‐controlled phase III trial of faldaprevir in combination with pegylated interferon a lf a‐2a and ribavirin in treatment‐experienced patients with chronic hepatitis C genotype‐1 infection. Hepatology 2013;48(S1):742A‐3A.
-
- Jacobson IM, Ferenci P, Foster GR, Jensen DM, Negro F, Mantry P, et al. STARTVerso3: a randomized, double‐blind, placebo controlled phase III trial of faldaprevir in combination with pegylated interferon alpha‐2a and ribavirin in treatment experienced patients with chronic hepatitis C genotype‐1 infection. Hepatology International 2014;8(1 Suppl):S213‐4.
STARTverso‐3 2013a3 {published data only}
-
- Asselah T, Zehnter E, Agarwal K, Sakai Y, Yatsuhashi H, Willems B, et al. Pharmacokinetic‐response analysis of faldaprevir in patients with chronic HCV genotype‐1 infection with prior relapse. Journal of Hepatology 2014;60(1 Suppl S):S458‐9.
-
- Berger K, Sarrazin C, Ferenci P, Jensen DM, Jacobson IM, Stern JO, et al. NS3 Q80K did not impact efficacy or treatment‐emergent resistance patterns in HCV genotype 1‐infected patients receiving faldaprevir+peginterferon/ribavirin in three phase III trials. Journal of Hepatology 2014;60(1 Suppl S):S497.
-
- Jacobson IM, Asselah T, Ferenci P, Foster GR, Jensen DM, Negro F, et al. STARTVerso3: a randomized, double‐blind, placebo‐controlled phase III trial of faldaprevir in combination with pegylated interferon a lf a‐2a and ribavirin in treatment‐experienced patients with chronic hepatitis C genotype‐1 infection. Hepatology 2013;48(S1):742A‐3A.
-
- Jacobson IM, Ferenci P, Foster GR, Jensen DM, Negro F, Mantry P, et al. STARTVerso3: a randomized, double‐blind, placebo controlled phase III trial of faldaprevir in combination with pegylated interferon alpha‐2a and ribavirin in treatment experienced patients with chronic hepatitis C genotype‐1 infection. Hepatology International 2014;8(1 Suppl):S213‐4.
STARTverso‐4 2015 {published data only}
-
- Dieterich D, Nelson M, Soriano V, Arasteh K, Guardiola JM, Rockstroh JK, et al. Faldaprevir and pegylated interferon alpha‐2a/ribavirin in individuals co‐infected with hepatitis C virus genotype‐1 and HIV. AIDS 2015;29(5):571‐81. [PUBMED: 25710287] - PubMed
Sulkowski 2013a {published data only}
-
- Sherman KE, Rockstroh JK, Dieterich DT, Soriano V, Girard PM, McCallister S, et al. Telaprevir combination with peginterferon alfa‐2A/ribavirin in HCV/HIV coinfected patients: 24‐week treatment interim analysis. Hepatology 2011;54(4 Suppl):1431A‐2A.
-
- Sulkowski MS, Sherman KE, Dieterich DT, Bsharat M, Mahnke L, Rockstroh JK, et al. Combination therapy with telaprevir for chronic hepatitis C virus genotype 1 infection in patients with HIV: a randomized trial. Annals of Internal Medicine 2013;159(2):86‐96. - PubMed
-
- Sulkowski MS, Sherman KE, Soriano V, Rockstroh J, Dieterich DT, Girard PM, et al. Telaprevir in combination with peginterferon alfa‐2a/ribavirin in HCV/HIV co‐infected patients: SVR24 final study results. Hepatology 2012;56(S1):219A.
Sulkowski 2013b {published data only}
-
- Sulkowski M, Pol S, Mallolas J, Fainboim H, Cooper C, Slim J, et al. Boceprevir versus placebo with pegylated interferon alfa‐2b and ribavirin for treatment of hepatitis C virus genotype 1 in patients with HIV: a randomised, double‐blind, controlled phase 2 trial. Lancet Infectious Diseases 2013;13(7):597‐605. - PubMed
Sulkowski 2013c {published data only}
-
- Kukolj G, Bethell R, Cartier M, Cote‐Martin A, Lagace L, Marguis M, et al. Characterization of HCV NS3 variants that emerged during virologic breakthrough and relapse from BI 201335 phase II SILEN‐C2 study in PEGIFN/RBV treatment‐experienced patients. Journal of Hepatology 2012;56(Suppl S2):S469.
-
- Sulkowski MS, Asselah T, Ferenc P, Stern JO, Kukolj G, Boecher WO, et al. Treatment with the second generation HCV protease inhibitor BI201335 results in high and consistent SVR rates ‐ results from silen‐C1 in treatment‐naive patients across different baseline factors. Hepatology 2011;54(Suppl S1):473A.
-
- Sulkowski MS, Asselah T, Lalezari J, Ferenci P, Fainboim H, Leggett B, et al. Faldaprevir combined with pegylated interferon alfa‐2a and ribavirin in treatment‐naïve patients with chronic genotype 1 HCV: SILEN‐C1 trial. Hepatology 2013;57(6):2143‐54. - PubMed
-
- Sulkowski MS, Ceasu E, Asselah T, Caruntu FA, Lalezari J, Ferenci P, et al. Silen‐C1: sustained virologic response (SVR) and safety of BI201335 combined with peginterferon alfa‐2a and ribavirin (P/R) in treatment‐naive patients with chronic genotype 1 HCV Infection. Journal of Hepatology 2011;54(Suppl 1):S27.
Sullivan 2012 {published data only}
-
- Sullivan GJ, Rodriques‐Torres M, Lawitz E, Poordad F, Kapoor M, Campbell A, et al. ABT‐267 combined with pegylated interferon alpha‐ 2a/ribavirin in genotype 1 (GT1) HCV‐infected treatment naive subjects: 12 week antiviral and safety analysis. Journal of Hepatology 2012;56(Suppl S2):S480.
Tanwandee 2012 {published data only}
-
- Tanwandee T, Luscombe C, Ewart G, Wilkinson J, Miller M, Peters MG, et al. High sustained viral response with a HCV p7 inhibitor, BIT225: antiviral activity and tolerability of BIT225 plus pegylated interferon alfa 2b and weight‐based ribavirin for 28 days in HCV treatment‐naive patients. Hepatology 2012;56(S1):1530A.
Tatum 2015a1 {published data only}
-
- McPhee F, Falk P, Fracasso P, Lemm J, Liu M, Kirk M, et al. Characterization of viral escape in HCV genotype 1‐infected patients treated with BMS‐791325 and pegylated interferon‐ALFA and ribavirin. Journal of Hepatology 2012;56(Suppl S2):S473.
-
- Tatum H, Thuluvath P, Lawitz E, Martorell CT, Demicco M, Cohen S, et al. A phase 2A study of BMS‐791325, an NS5B polymerase inhibitor, with peginterferon alfa‐2A and ribavirin in treatment‐naive patients with genotype 1 chronic hepatitis C infection. Journal of Hepatology 2012;56(Suppl S2):S460.
-
- Tatum H, Thuluvath PJ, Lawitz E, Martorell C, Demicco M, Cohen S, et al. A randomized, placebo‐controlled study of the NS5B inhibitor beclabuvir with peginterferon/ribavirin for HCV genotype 1. Journal of Viral Hepatitis 2015;22(8):658‐64. - PubMed
-
- Tatum HA, Thuluvath PJ, Lawitz E, Martorell C, Cohen SM, Rustgi VK, et al. Safety and efficacy of BMS‐791325, a non‐nucleoside NS5B polymerase inhibitor, combined with peginterferon alfa‐2a and ribavirin in treatment‐naive patients infected with hepatitis C virus genotype 1. Hepatology 2013;58(S1):759A‐60A.
Tatum 2015a2 {published data only}
-
- McPhee F, Falk P, Fracasso P, Lemm J, Liu M, Kirk M, et al. Characterization of viral escape in HCV genotype 1‐infected patients treated with BMS‐791325 and pegylated interferon‐ALFA and ribavirin. Journal of Hepatology 2012;56(Suppl S2):S473.
-
- Tatum H, Thuluvath P, Lawitz E, Martorell CT, Demicco M, Cohen S, et al. A phase 2A study of BMS‐791325, an NS5B polymerase inhibitor, with peginterferon alfa‐2A and ribavirin in treatment‐naive patients with genotype 1 chronic hepatitis C infection. Journal of Hepatology 2012;56(Suppl S2):S460.
-
- Tatum H, Thuluvath PJ, Lawitz E, Martorell C, Demicco M, Cohen S, et al. A randomized, placebo‐controlled study of the NS5B inhibitor beclabuvir with peginterferon/ribavirin for HCV genotype 1. Journal of Viral Hepatitis 2015;22(8):658‐64. - PubMed
-
- Tatum HA, Thuluvath PJ, Lawitz E, Martorell C, Cohen SM, Rustgi VK, et al. Safety and efficacy of BMS‐791325, a non‐nucleoside NS5B polymerase inhibitor, combined with peginterferon alfa‐2a and ribavirin in treatment‐naive patients infected with hepatitis C virus genotype 1. Hepatology 2013;58(S1):759A‐60A.
Vierling 2011 {published data only}
-
- Vierling JM, Poordad FF, Lawitz E, Ghalib RH, Lee WM, Ravendhran N, et al. Once daily narlaprevir (NVR; SCH 900518) and ritonavir (RTV) in combination with peginterferon alfa‐2B/ribavirin (PR) for 12 weeks plus 12 weeks pr in treatment‐naïve patients with HCV genotype 1 (G1): SVR results from next‐1, a phase 2 study. Hepatology 2011;54(Suppl S1):1437A‐8A.
Villano 2007 {published data only}
-
- Villano S, Raible D, Harper D, Chandra P, Bazisotto L, Bichier G. Phase 1 evaluation of antiviral activity of the non‐nucleoside polymerase inhibitor, HCV‐796, in combination with different pegylated interferons in treatment‐naive patients with chronic HCV. Hepatology 2007;46(4 Suppl 1):815A.
Vince 2014 {published data only}
-
- Vince B, Hill JM, Lawitz EJ, O'Riordan W, Webster LR, Gruener DM, et al. A randomized, double‐blind, multiple‐dose study of the pan‐genotypic NS5A inhibitor samatasvir in patients infected with hepatitis C virus genotype 1, 2, 3 or 4. Journal of Hepatology 2014;60(5):920‐7. - PubMed
Wedemeyer 2013 {published data only}
-
- Chen YC, Moreira S, Ipe D, Kulkarni R, Passe S, Zhu Y, et al. The effect of host il28b genotype and mericitabine (MCB; RG7128) dose on early viral kinetics following administration of MCB plus pegifn alpha‐2a plus ribavirin (P/R) in patients with chronic hepatitis C (CHC). Hepatology 2011;54(Suppl S1):553A.
-
- Jensen DM, Wedemeyer H, Herring RW, Ferenci P, Ma MM, Zeuzem S, et al. High rates of early viral response, promising safety profile and lack of resistance‐related breakthrough in HCV GT 1/4 patients treated with RG7128 plus PegIFN alfa‐2a (40KD)/RBV: planned week 12 interim analysis from the PROPEL study. Hepatology 2010;52(Suppl S1):360A.
-
- Wedemeyer H, Jensen D, Herring R, Ferenci P, Ma MM, Zeuzem S, et al. Efficacy and safety of mericitabine (MCB) in combination with Peg‐Ifn alpha‐2A/RBV in G1/4 treatment naive HCV patients: final analysis from the PROPEL study. Journal of Hepatology 2012;56(Suppl S2):S481‐2.
-
- Wedemeyer H, Jensen D, Herring R, Ferenci P, Ma MM, Zeuzem S, et al. PROPEL: a randomized trial of mericitabine plus peginterferon alpha‐2a/ribavirin therapy in treatment‐naive HCV genotype 1/4 patients. Hepatology 2013;58(2):524‐37. - PubMed
Wilfret 2013 {published data only}
-
- Spreen W, Wilfret DA, Bechtel J, Adkison KK, Lou Y, Jones L, et al. GSK2336805 HCV NS5A inhibitor demonstrates potent antiviral activity in chronic genotype 1 infection; results from a first time in human (FTIH) single and repeat dose study. Hepatology 2011;54(Suppl S1):400A‐1A.
-
- Wilfret DA, Walker J, Adkinson KK, Jones LA, Lou Y, Gan J, et al. Safety, tolerability, pharmacokinetics, and antiviral activity OFGSK2336805, an inhibitor of hepatitis C virus (HCV) NA5A, in healthy subjects and subjects chronically infected with HCV genotype 1. Antimicrobial Agents and Chemotherapy 2013;57(10):5037‐44. - PMC - PubMed
Younossi 2015 {published data only}
-
- Younossi Z, Stepanova M, Pol S, Bronowicki JP, Carrieri P, Bourliere M. The impact of ledipasvir (LDV)/sofosbuvir (SOF) combination on health‐related quality of life (HRQL) and patient‐reported outcomes (PROS) in cirrhotic patients with chronic hepatitis C (CH‐C): the SIRIUS study. Journal of Hepatology 2015;62(Suppl S2):S591.
Zeuzem 2011a {published data only}
-
- Berg T, Andreone P, Pol S, Roberts SK, Younossi ZM, Diago M, et al. Predictors of virologic response with telaprevir‐based combination treatment in HCV genotype 1‐infected patients with prior peginterferon/ribavirin treatment failure: post‐hoc analysis of the phase III REALIZE study. Hepatology 2011;54(S1):375A‐6A.
-
- Meyer S, Dierynck I, Ghys A, Beumont M, Daems B, Baelen B, et al. Characterization of telaprevir treatment outcomes and resistance in patients with prior treatment failure: results from the REALIZE trial. Hepatology 2012;56(6):2106‐15. - PubMed
-
- Foster GR, Jacobson IM, Dore GJ, Fried M, Manns M, Marcellin P, et al. Simeprevir (TMC435) with peginterferon/ribavirin for treatment of chronic HCV genotype 1 infection in treatment‐naive European patients in the QUEST‐1 and QUEST‐2 phase III trials. Journal of Hepatology 2014;60(1 Suppl 1):S456.
-
- Foster GR, Zeuzem S, Andreone P, Pol S, Lawitz EJ, Diago M, et al. Subanalyses of the telaprevir lead‐in arm in the REALIZE study: response at week 4 is not a substitute for prior null response categorization. Journal of Hepatology 2011;54(Suppl 1):S3‐S4.
-
- Foster GR, Zeuzem S, Andreone P, Pol S, Lawitz EJ, Diago M, et al. Sustained virologic response rates with telaprevir by response after 4 weeks of lead‐in therapy in patients with prior treatment failure. Journal of Hepatology 2013;58(3):488‐94. - PubMed
Zeuzem 2014a {published data only}
-
- Liu Y, Larsen L, Bourliere M, Baran R, Juday T. Ombitasvir/paritaprevir/ritonavir and dasabuvir with ribavirin (RBV) has mild impact on health‐related quality of life (HRQoL) compared with placebo during 12‐week treatment in treatment‐experienced adults with chronic hepatitis C (CHC). Journal of Hepatology 2015;62(Suppl S2):S661.
-
- Zeuzem S, Jacobson IM, Baykal T, Marinho RT, Poordad F, Bourliere M, et al. Retreatment of HCV with ABT‐450/r‐ombitasvir and dasabuvir with ribavirin. New England Journal of Medicine 2014;370(17):1604‐14. - PubMed
References to studies excluded from this review
AGATE‐I 2015 {published data only}
-
- Asselah T, Hassanein TI, Qaqish RB, Feld JJ, Hezode C, Zeuzem S, et al. Efficacy and safety of ombitasvir/paritaprevir/ritonavir co‐administered with ribavirin in adults with genotype 4 chronic hepatitis C infection and cirrhosis (AGATE‐I). Hepatology 2015;62(Suppl S1):563A‐4A. - PubMed
ALLY 2015 {published data only}
-
- Wyles D, Ruane P, Sulkowski M, Dieterich D, Luetkemeyer A, Morgan T, et al. Daclatasvir in combination with sofosbuvir for HIV/HCV coinfection: ALLY‐2 study. Topics in Antiviral Medicine 2015; Vol. 23, issue e‐1:62.
ANNAPURNA 2013 {published data only}
-
- Jensen DM, Brunda M, Elston R, Gane EJ, George J, Glavini K, et al. Interferon‐free regimen containing setrobuvir (STV) in combination with ritonavir‐boosted danoprevir (DNVr) and ribavirin (R) with or without mericitabine (MCB) in HCV genotype (G)1 treatment‐naive patients: interim SVR4 results from the ANNAPURNA study. Hepatology 2013;58(S1):741A.
APRICOT 2004 {published data only}
-
- APRICOT Study Group. APRICOT Study evaluated. Surprisingly high virologic response in HIV‐HCV coinfection. MMW Fortschritte der Medizin 2004;146(Spec No 1):74‐5. - PubMed
ATOMIC 2013 {published data only}
-
- Kowdley KV, Lawitz E, Crespo I, Hassanein T, Davis MN, Demicco M, et al. Sofosbuvir with pegylated interferon alfa‐2a and ribavirin for treatment‐naive patients with hepatitis C genotype‐1 infection (ATOMIC): an open‐label, randomised, multicentre phase 2 trial. Lancet 2013;381(9883):2100‐7. - PubMed
ATTAIN 2015 {published data only}
-
- Reddy KR, Zeuzem S, Zoulim F, Weiland O, Horban A, Stanciu C. A phase III randomised, double‐blind study to evaluate the efficacy, safety and tolerability of simeprevir vs telaprevir in combination with pegylated interferon and ribavirin in chronic hepatitis C virus genotype 1 treatment‐experienced patients: the ATTAIN study. Hepatology International 2014;8(Suppl 1):S397.
-
- Reddy KR, Zeuzem S, Zoulim F, Weiland O, Horban A, Stanciu C, et al. Simeprevir versus telaprevir with peginterferon and ribavirin in previous null or partial responders with chronic hepatitis C virus genotype 1 infection (ATTAIN): A randomised, double‐blind, non‐inferiority phase 3 trial. Lancet Infectious Diseases 2015;15(1):27‐35. - PubMed
-
- Scott J, Cerri K, Sbarigia U, Corbett C, Fu M, Jessner W. Simeprevir with PegIFN/ribavirin for chronic HCV infection shortens time with patient reported symptoms and impairment in QoL: ATTAIN study results. Journal of Viral Hepatitis 2014;21(Suppl S2):25.
-
- Scott J, Corbett C, Gilles L, Wan G, Sbarigia U, Jessner W. Impact of simeprevir versus telaprevir triple therapy for chronic HCV infection on patient‐reported outcomes in prior non‐responders to peginterferon/ribavirin results from the phase III attain study. Value in Health 2014;17(7):A682. - PubMed
AVIATOR 2015 {published data only}
-
- Poordad F, Agarwal K, Younes Z, Cohen D, Xie WG, Podsadecki T. Low relapse rate leads to high concordance of sustained virologic response (SVR) at 12 weeks with SVR at 24 weeks after treatment with abt‐450/ritonavir, ombitasvir, and dasabuvir plus ribavirin in subjects with chronic hepatitis C virus genotype 1 infection in the aviator study. Clinical Infectious Diseases 2015;60(4):608‐10. - PubMed
-
- Szeinbach SL, Baran RW. Treatment satisfaction in clinical trial (AVIATOR) patients treated with interferon‐free, oral DAA regimens: external validation of the hepatitis C virus (HCV) treatment satisfaction (HCVTSat) instrument. Gastroenterology 2014;146(5 Suppl 1):S‐977.
Basu 2014b {published data only}
-
- Basu P, Shah N, Aloysius M. Simeprevir and sofosbuvir with modified doses of ribavirin (RBV) therapy on telaprevir‐experienced, coinfected (with HIV) cirrhotics with chronic hepatitis C (CHC): a randomized, open‐label, clinical pilot study, STOP C, interim results. American Journal of Gastroenterology 2014;109(Suppl 2):S177.
Bathgate 2011 {published data only}
-
- Bathgate A. Boceprevir for previously treated chronic hepatitis C virus genotype I infection. Journal of the Royal College of Physicians of Edinburgh 2011;41(2):122‐3. - PubMed
Bognar 2011 {published data only}
-
- Bognar F. Projecting the clinical impact of therapeutic regimens including boceprevir in previously untreated adult subjects with chronic hepatitis C genotype 1. Journal of Gastroenterology and Hepatology 2011;26(Suppl 5):19.
Bourgeois 2015 {published data only}
-
- Bourgeois S, Nevens F, Moreno C, Vlierberghe H, Arasteh K, Horsmans Y, et al. Efficacy safety and pharmacokinetics of 12 weeks of simeprevir in combination with TMC647055 ritonavir and JNJ 56914845 in genotype 1 hepatitis C virus infected patients. Hepatology International 2015;9(1 Suppl):S57‐8.
Chandra 2006b {published data only}
-
- Chandra P, Raible D, Moyer L, Harper D, Speth J, Villano S, et al. Safety and pharmacokinetics of the non‐nucleoside polymerase inhibitor, HCV‐796: results of a randomized, double‐blind, placebo‐controlled, ascending single‐dose study in healthy subjects. Journal of Hepatology 2006;44(2 Suppl):S208‐9.
CONCISE 2013 {published data only}
-
- Nelson DR, Poordad F, Feld JJ, Fried MW, Jacobson IM, Pockros PJ, et al. High SVR rates (SVR4) for 12‐week total telaprevir combination therapy in IL28B CC treatment‐naives and prior relapsers with G1 chronic hepatitis C: concise interim analysis. Journal of Hepatology 2013;58(Suppl S1):S362.
COSMOS 2014 {published data only}
-
- Lawitz E, Sulkowski MS, Ghalib R, Rodriguez‐Torres M, Younossi ZM, Corregidor A, et al. Simeprevir plus sofosbuvir, with or without ribavirin, to treat chronic infection with hepatitis C virus genotype 1 in non‐responders to pegylated interferon and ribavirin and treatment‐naive patients: the COSMOS randomised study. Lancet 2014;384(9956):1756‐65. [PUBMED: 25078309] - PubMed
C‐SURFER 2015 {published data only}
-
- Elbasha EH, Ferrante S, Agarwal E, Greaves W, Nwankwo C. Projected clinical and economic impact of grazoprevir (GZR, MK‐5172)/elbasvir (EBR, MK‐8742) for chronic HCV genotype 1 infection in chronic kidney disease. Value in Health 2015;18(7):A510.
-
- Roth D, Nelson D, Bruchfeld A, Liapakis A, Silva M, Monsour Jr H, et al. C‐surfer: grazoprevir plus elbasvir in treatment‐naive and treatment‐experienced patients with hepatitis C virus genotype 1 infection and chronic kidney disease. Journal of Hepatology 2015;62(Suppl S2):S263‐4. - PubMed
-
- Roth D, Nelson DR, Bruchfeld A, Liapakis A, Silva M, Monsour H, et al. Grazoprevir plus elbasvir in treatment‐naive and treatment‐experienced patients with hepatitis C virus genotype 1 infection and stage 4‐5 chronic kidney disease (the C‐SURFER study): a combination phase 3 study. Lancet 2015;386(10003):1537‐45. - PubMed
C‐WORTHY 2015 {published data only}
-
- Lawitz E, Gane E, Pearlman B, Tam E, Ghesquiere W, Guyader D, et al. Efficacy and safety of 12 weeks versus 18 weeks of treatment with grazoprevir (MK‐5172) and elbasvir (MK‐8742) with or without ribavirin for hepatitis C virus genotype 1 infection in previously untreated patients with cirrhosis and patients with previous null response with or without cirrhosis (C‐WORTHY): a randomised, open‐label phase 2 trial. Lancet 2015;385(9973):1075‐86. - PubMed
-
- Lawitz E, Vierling JM, Murillo A, Kugelmas M, Gerstoft J, Winkle P, et al. High efficacy and safety of the all‐oral combination regimen, MK‐5172/MK‐8742 +/‐ RBV for 12 weeks in HCV genotype 1 infected patients: the C‐WORTHY study. Hepatology 2013;48(S1):244A‐5A.
-
- Sulkowski M, Hezode C, Gerstoft J, Vierling JM, Mallolas J, Pol S, et al. Efficacy and safety of 8 weeks versus 12 weeks of treatment with grazoprevir (MK‐5172) and elbasvir (MK‐8742) with or without ribavirin in patients with hepatitis C virus genotype 1 mono‐infection and HIV/hepatitis C virus co‐infection (C‐WORTHY): a randomised, open‐label phase 2 trial. Lancet 2015;385(9973):1087‐97. - PubMed
-
- Sulkowski M, Mallolas J, Bourliere M, Gerstoft J, Shibolet O, Nahass R, et al. On‐treatment viral response to MK‐5172/MK‐8742 +/‐ RBV for 12 weeks in HCV/HIV‐coinfected patients. Topics in Antiviral Medicine 2014; Vol. 22, issue e‐1:324‐5.
Di Bisceglie 2014 {published data only}
-
- Bisceglie AM, Sulkowski M, Gane E, Jacobson IM, Nelson D, DeSouza C, et al. VX‐222, a non‐nucleoside NS5B polymerase inhibitor, in telaprevir‐based regimens for genotype 1 hepatitis C virus infection. European Journal of Gastroenterology & Hepatology 2014;26(7):761‐73. - PubMed
Dore 2014 {published data only}
-
- Dore GJ, Jacobson IM, Foster GR, Fried M, Manns M, Marcellin P, et al. Simeprevir (TMC435) with peg‐interferon/ribavirin for treatment of chronic HCV genotype 1 infection in treatment naive patients: efficacy in patients with genotype 1b HCV in the QUEST‐1 and‐2 phase III trials. Hepatology International 2014;8(1 Suppl 1):S195.
Dusheiko 2015 {published data only}
-
- Dusheiko GM, Manns MP, Vierling JM, Reddy KR, Sulkowski MS, Kwo PY, et al. Safety and tolerability of grazoprevir/elbasvir in patients with chronic hepatitis C (HCV) infection: integrated analysis of phase 2‐3 trials. Hepatology 2015;62(Suppl S1):562A.
Ferenci 2014 {published data only}
-
- Ferenci P, Bernstein D, Lalezari J, Cohen D, Luo Y, Cooper C, et al. ABT‐450/r‐ombitasvir and dasabuvir with or without ribavirin for HCV. New England Journal of Medicine 2014;370(21):1983‐92. - PubMed
Ferrante 2011a {published data only}
-
- Ferrante SA, Chhatwal J, Dasbach EJ, Sniukiene V, Poordad F, Bronowicki JP. Projecting the clinical impact of therapeutic regimens including boceprevir in previously untreated adult subjects with chronic hepatitis C genotype 1. Gastroenterology 2011;140(5 Suppl 1):S909.
Ferrante 2011b {published data only}
-
- Ferrante SA, Chhatwal J, Elbasha E, Dasbach EJ, Khoury A, Poordad F, et al. Cost‐effectiveness of boceprevir based regimens in previously untreated adult subjects with chronic hepatitis C genotype 1. Hepatology 2011;54(4 Suppl):795A‐796A.
Ferrante 2013 {published data only}
Foster 2010 {published data only}
-
- Foster GF, Dusheiko G, Hezode C, Marcellin P, McHutchinson J, Beumont M, et al. Final results from telaprevir phase II studies in genotype 1 treatment‐naive or experienced subjects with chronic hepatitis C. Gut 2010;59(Suppl 2):A5.
FOURward 2014 {published data only}
-
- Sulkowski MS, Flamm SL, Kayali Z, Lawitz E, Kwo PY, McPhee F, et al. Short‐duration therapy with daclatasvir/asunaprevir/beclabuvir fixed‐dose combination plus sofosbuvir in patients with chronic hepatitis C genotype 1 (FOURward study). Hepatology 2015;62(Suppl S1):556A.
FUSION 2013 {published data only}
-
- Jacobson IM, Gordon SC, Kowdley KV, Yoshida EM, Rodriguez‐Torres M, Sulkowski MS, et al. Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment options. New England Journal of Medicine 2013;368(20):1867‐77. - PubMed
Gardner 2014b {published data only}
-
- Gardner S, Cutrell AMY, Elko‐Simms C, Adkison K, Hamatake R, Walker J, et al. A double‐blind, randomized, placebo‐controlled study to assess the safety, antiviral activity and pharmacokinetics of GSK2336805 when given as monotherapy and in combination with peginterferon alfa‐2a and ribavirin in hepatitis C virus genotype 1‐infected treatment‐naive subjects. Liver International 2014;34(6):e89‐95. - PubMed
HCVerso 1 2014 {published data only}
-
- Sarrazin C, Castelli F, Puoti M, Shiffman ML, Preotescu L, Forns X, et al. HCVerso1: a phase III study of faldaprevir (FDV) plus deleobuvir (DBV) and ribavirin (RBV) for chronic HCV genotype (GT)‐1b infection in treatment‐naive patients. Hepatology 2014;59(Suppl S1):1150A. [DOI: ]
HCVerso 2 2014 {published data only}
-
- Nelson DR, Andreone P, Colombo M, Calinas F, Olveira A, Delwaide J, et al. HCVerso2: a phase III study of faldaprevir (FDV) plus deleobuvir (DBV) and ribavirin (RBV) for chronic HCV genotype (GT)‐1b infection in treatment‐naive patients including those ineligible for pegylated interferon (PegIFN). Hepatology 2014;59(Suppl S1):1155A‐6A. [DOI: ]
ION‐3 2014 {published data only}
-
- Kowdley KV, Gordon SC, Reddy KR, Rossaro L, Bernstein DE, Lawitz E, et al. Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. New England Journal of Medicine 2014;370(20):1879‐88. - PubMed
Jacobson 2013 {published data only}
-
- Jacobson IM, Dore GJ, Foster GR, Fried MW, Manns MP, Marcellin P, et al. Simeprevir (TMC435) with peginterferon/ribavirin for treatment of chronic HCV genotype 1 infection in treatment‐naïve patients: efficacy in difficult‐to‐treat patient sub‐populations in the QUEST 1 and 2 phase III trials. Hepatology 2013;58(S1):756A‐7A.
Kawada 2015 {published data only}
-
- Kawada N, Suzuki F, Karino Y, Chayama K, Itoh Y, Okanoue T, et al. Efficacy, safety and pharmacokinetics of grazoprevir (MK‐5172) and elbasvir (MK‐8742) In hepatitis C genotype 1 infected non‐cirrhotic Japanese patients (phase 2 portion in phase 2/3 combined study). Hepatology 2015;62(Suppl S1):559A.
Liu 2015b {published data only}
-
- Liu Y, Bernstein D, Larsen L, Luo Y, Xie Y, Juday T. Ribavirin does not impact health‐related quality of life (HRQoL) in patients on ombitasvir/paritaprevir/ritonavir and dasabuvir at the end of 12‐week treatment in treatment‐naive adults with genotype 1a (GT1a) chronic hepatitis C. Value in Health 2015;18(3):A227.
Lok 2010 {published data only}
-
- Lok AS, Gardiner DF, Lawitz E, Martorell C, Everson GT, Ghalib RH, et al. Combination therapy with BMS‐790052 and BMS‐650032 alone or with pegIFN/RBV results in undetectable HCV RNA through 12 weeks of therapy in HCV genotype 1 null responders. Hepatology 2010;52(Suppl S1):877A.
Lok 2011 {published data only}
-
- Lok A, Gardiner D, Lawitz E, Martorell C, Everson G, Ghalib R, et al. Quadruple therapy with BMS‐790052, BMS‐650032 and PEG‐IFN/RBV for 24 weeks results in 100% SVR12 in HCV genotype 1 null responders. Journal of Hepatology 2011;54(Suppl 1):S536.
Lok 2012a {published data only}
-
- Lok A, Gardiner D, Hezode C, Lawitz E, Bourliere M, Everson G, et al. Confirmation that quadruple therapy with daclatasvir (NS5A inhibitor), asunaprevir (NS3 inhibitor) and peginterferon/ribavirin results in high rate of svr4 in HCV genotype 1 null responders. Journal of Hepatology 2012;56(Suppl 2):S557.
Lok 2012b {published data only}
-
- Lok AS, Gardiner DF, Hezode C, Lawitz E, Bourliere M, Everson GT, et al. Sustained virologic response in chronic HCV genotype (GT) 1‐infected null responders with combination of daclatasvir (DCV; NS5A Inhibitor) and asunaprevir (ASV; NS3 Inhibitor) with or without peginterferon alfa‐2a/ribavirin (PEG/RBV). Hepatology 2012;56(S1):230A‐1A.
Lok 2014 {published data only}
-
- Lok AS, Gardiner DF, Hezode C, Lawitz EJ, Bourliere M, Everson GT, et al. Randomized trial of daclatasvir and asunaprevir with or without PegIFN/RBV for hepatitis C virus genotype 1 null responders. Journal of Hepatology 2014;60(3):490‐9. - PubMed
MALACHITE‐I 2016 {published data only}
-
- Conway B, Janczewska EWA, Luo YAN, Curescu M, Greenbloom S, Streinu‐Cercel A, et al. Malachite‐I: phase 3b trial of ombitasvir/paritaprevir/r and dasabuvir plus /‐ ribavirin or telaprevir plus peginterferon/ribavirin in treatment‐naive adults with HCV genotype 1. Gastroenterology 2015;148(4 Suppl 1):S1001‐S2.
-
- Dore GJ, Conway B, Luo YAN, Janczewska EWA, Knysz B, Liu YAN, et al. Efficacy and safety of ombitasvir/paritaprevir/r and dasabuvir compared to IFN‐containing regimens in genotype 1 HCV patients: the malachite‐I/II trials. Journal of Hepatology 2016;64(1):19‐28. - PubMed
-
- Liu Y, Luo Y, Liu X, Sullivan D, Juday TR, Conway B, et al. Better work productivity and activity in patients on ombitasvir/ paritaprevir/ritonavir and dasabuvir with or without ribavirin (3D+RBV or 3D) in treatment‐naive adults with genotype 1 (GT1) chronic hepatitis C. Hepatology 2015;62(Suppl S1):802A.
MALACHITE‐II 2016 {published data only}
-
- Dore G, Knysz B, Luo Y, Janczewska E, Streinu‐Cercel A, Caruntu FA, et al. Malachite‐II: phase 3B trial of ombitasvir/paritaprevir/R and dasabuvir + ribavirin or telaprevir + peginterferon/ribavirin in peginterferon/ribavirin treatment‐experienced adults with HCV genotype 1. Journal of Hepatology 2015;62(Suppl S2):S656‐7.
-
- Dore GJ, Conway B, Luo YAN, Janczewska EWA, Knysz B, Liu YAN, et al. Efficacy and safety of ombitasvir/paritaprevir/r and dasabuvir compared to IFN‐containing regimens in genotype 1 HCV patients: the malachite‐I/II trials. Journal of Hepatology 2016;64(1):19‐28. - PubMed
Manns 2014b {published data only}
-
- Manns MP, McCone J Jr, Davis MN, Rossaro L, Schiff E, et al. Overall safety profile of boceprevir plus peginterferon alfa‐2b and ribavirin in patients with chronic hepatitis C genotype 1: a combined analysis of 3 phase 2/3 clinical trials. Liver International 2014;34(5):707‐19. - PubMed
Manns 2015 {published data only}
-
- Manns M, Forns X, Samuel D, Denning J, Arterburn S, Brandt‐Sarif T, et al. Ledipasvir/sofosbuvir with ribavirin is safe and efficacious in decompensated and post liver transplantation patients with HCV infection: preliminary results of the prospective SOLAR 2 trial. Journal of Hepatology 2015;62(Suppl S2):S187‐8.
Mendez 2014 {published data only}
-
- Mendez P, Suzuki Y, Ikeda K, Toyota J, Karino Y, Kawakami Y, et al. Safety of asunaprevir (ASV) in the all‐oral dual combination with daclatasvir (DCV) or in combination with peginterferon/ribavirin (pegIFN/RBV) for chronic HCV infection. Hepatology International 2014;59(Suppl S1):S186‐7.
Mizokami 2015 {published data only}
-
- Mizokami M, Yokosuka O, Takehara T, Sakamoto N, Korenaga M, Mochizuki H, et al. Ledipasvir and sofosbuvir fixed‐dose combination with and without ribavirin for 12 weeks in treatment‐naive and previously treated Japanese patients with genotype 1 hepatitis C: an open‐label, randomised, phase 3 trial. Lancet Infectious Diseases 2015;15(6):645‐53. - PubMed
Molina 2015 {published data only}
-
- Molina JM, Orkin C, Iser DM, Zamora FX, Nelson M, Stephan C, et al. Sofosbuvir plus ribavirin for treatment of hepatitis C virus in patients co‐infected with HIV (PHOTON‐2): a multicentre, open‐label, non‐randomised, phase 3 study. Lancet 2015;385(9973):1098‐106. - PubMed
Muir 2011 {published data only}
-
- Muir AJ, Poordad FF, McHutchison JG, Shiffman ML, Berg T, Ferenci P, et al. Retreatment with telaprevir combination therapy in hepatitis C patients with well‐characterized prior treatment response. Hepatology 2011;54(5):1538‐46. - PubMed
Muir 2015 {published data only}
-
- Muir A, Poordad F, Lalezari J, Dore G, Hezode C, Ramji A, et al. Unity‐2: daclatasvir/asunaprevir/beclabuvir +/‐ RBV for HCV genotype 1 with cirrhosis. Topics in Antiviral Medicine 2015;23(e‐1):301.
-
- Muir A, Poordad F, Lalezari J, Everson G, Dore G, Kwo P, et al. Fixed dose oral combination therapy with daclatasvir asunaprevir beclabuvir‐ribavirin for patients with chronic HCV genotype 1 infection and compensated cirrhosis unity 2 phase 3 results. Hepatology International 2015;9(1 Suppl 1):S47.
-
- Muir AJ, Poordad F, Lalezari J, Everson G, Dore GJ, Herring R, et al. Daclatasvir in combination with asunaprevir and beclabuvir for hepatitis C virus genotype 1 infection with compensated cirrhosis. JAMA 2015;313(17):1736‐44. - PubMed
NEUTRINO 2013 {published data only}
-
- Lawitz E, Mangia A, Wyles D, Rodriguez‐Torres M, Hassanein T, Gordon Stuart C, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. New England Journal of Medicine 2013;368(20):1878‐87. - PubMed
Nishiguchi 2014b {published data only}
-
- Nishiguchi S, Sakai Y, Kuboki M, Tsunematsu S, Urano Y, Sakamoto W, et al. Safety and efficacy of faldaprevir with pegylated interferon alfa‐2a and ribavirin in Japanese patients with chronic genotype‐1 hepatitis C infection. Liver International 2014;34(1):78‐88. - PubMed
Nomura 2014 {published data only}
-
- Nomura H, Miyagi Y, Tanimoto H, Kawano A, Yamashita N. Weight loss during telaprevir‐based triple therapy due to telaprevir‐induced appetite loss. Internal Medicine 2014;53(22):2567‐73. - PubMed
NUCLEAR 2013 {published data only}
-
- Lawitz EJ, Rodriguez‐Torres M, Denning J, Mathias A, Mo H, Gao B, et al. All‐oral therapy with nucleotide inhibitors sofosbuvir and GS‐0938 for 14 days in treatment‐naive genotype 1 hepatitis C (nuclear). Journal of Viral Hepatitis 2013;20(10):699‐707. - PubMed
OPTIMIST‐1 2015 {published data only}
-
- Kwo P, Gitlin N, Nahass R, Bernstein D, Rojter S, Schiff E, et al. A phase 3, randomised, open‐label study to evaluate the efficacy and safety of 8 and 12 weeks of simeprevir (SMV) plus sofosbuvir (SOF) in treatment‐naive and ‐experienced patients with chronic HCV genotype 1 infection without cirrhosis: OPTIMIST‐1. Journal of Hepatology 2015;62(Suppl S2):S270.
OPTIMIZE 2013 {published data only}
-
- Buti M, Agarwal K, Horsmans Y, Sievert W, Zeuzem S, Nyberg L, et al. Efficacy of telaprevir dosed twice daily versus every 8 hours by IL28B genotype: results from phase III OPTIMIZE study. Journal of Hepatology 2013;58(Suppl1):S326.
Poordad 2014 {published data only}
-
- Poordad F, Hezode C, Trinh R, Kowdley KV, Zeuzem S, Agarwal K, et al. ABT‐450/r‐ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrhosis. New England Journal of Medicine 2014;370(21):1973‐82. - PubMed
-
- Wedwemeyer H, Berg T, Flamm SL, Foster GR, Craxi A, Larrey D, et al. Improvement in liver function and non‐invasive estimates of liver fibrosis 48 weeks after treatment with ombitasvir/paritaprevir/r, dasabuvir and ribavirin in HCV genotype 1 patients with cirrhosis. Journal of Hepatology 2015;62(Suppl S2):S637‐8.
Proulx 2008 {published data only}
-
- Proulx L, Bourgault B, Chauret N, Larouche R, Tanguay M, Thibert R. Results of a safety, tolerability and pharmacokinetic phase I study of VCH‐916, a novel polymerase inhibitor for HCV, following single ascending doses in healthy volunteers. Journal of Hepatology 2008;48(Suppl S2):S320.
Reddy 2011 {published data only}
-
- Reddy KR, Nunes F, Balart LA, Sjogren R, Pedicone L, Burroughs M, et al. An evaluation of neutropenia in the pivotal studies of boceprevir (BOC) plus PegInterferon alfa‐2B/ribavirin (PR). Hepatology 2011;54(4 Suppl):814A.
Serfaty 2012 {published data only}
-
- Carosi G, Colombo M, Spinetti A, Zaltron S, Serfaty L, Marcellin P, et al. Impact of insulin resistance on antiviral efficacy of telaprevir‐based regimen in treatment‐naive genotype 1 HCV: results from C208 sub‐analysis. Digestive and Liver Disease 2011;43(Suppl 2):S84.
-
- Colombo M, Rumi MG, Carosi G, Puoti M, Marcellin P, Forns X, et al. Telaprevir (TVR) q8h or q12h combined with either peginterferon (PEG‐IFN, P) alfa‐2a or alfa‐2b and ribavirin (RBV, r) in treatment‐naive genotype 1 hepatitis C: final results of the randomized, open‐label, multicenter phase 2 study C208. Digestive and Liver Disease 2010;42(Suppl 1):S6‐7.
-
- Forns X, Marcellin P, Ferenci P, Goser T, Nevens F, Carosi G, et al. On‐treatment response‐guided therapy with telaprevir q8h or q12h combined with peginterferon alfa‐2a or peginterferon alfa‐2b and ribavirin in treatment‐naive genotype 1 hepatitis C (study c208). Journal of Hepatology 2010;52(Suppl S1):S26.
-
- Forns X, Marcellin P, Goeser T, Ferenci P, Nevens F, Carosi G, et al. Phase 2 study of telaprevir administered q8h or q12h with peginterferon‐alfa‐2a or ‐alfa‐2b and ribavirin in treatment‐naive subjects with genotype 1 hepatitis C: week 4 interim results. Hepatology 2008;48(4):1136A‐7A.
-
- Marcellin P, Forns X, Goeser T, Ferenci P, Nevens F, Carosi G, et al. On‐treatment response‐guided therapy with telaprevir Q8h or Q12h combined with peginterferon alfa‐2a or peginterferon alfa‐2b and ribavirin in treatment‐naive genotype 1 hepatitis C (Study C208). Gastroenterology 2010;138(5 Suppl 1):S847.
Sulkowski 2011 {published data only}
-
- Sulkowski MS, Poordad F, Manns MP, Bronowicki JP, Reddy KR, Harrison SA, et al. Anemia during treatment with peginterferon alfa‐2b/ribavirin with or without boceprevir is associated with higher SVR rates: analysis of previously untreated and previous‐treatment‐failure patients. Journal of Hepatology 2011;54(Suppl 1):S194‐5.
Sulkowski 2012a {published data only}
-
- Sulkowski MS, Roberts S, Afdhal N, Andreone P, Diago M, Pol S, et al. Ribavirin dose modification in treatment‐naive and previously treated patients who received telaprevir combination treatment: no impact on sustained virologic response in phase 3 studies. Journal of Hepatology 2012;56(Suppl S2):S459‐60.
Sulkowski 2012b {published data only}
-
- Sulkowski MS, Rodriguez‐Torres M, Lawitz E, Shiffman ML Pol S, Herring RW, et al. Complete SVR4 rates in treatment‐naive HCV genotype 1a and 1b patients who achieved vRVR with an interferon‐free all‐oral regimen. Hepatology 2012;56(S1):298A.
Sulkowski 2013d {published data only}
-
- Sulkowski M, Bourliere M, Bronowicki JP, Streinu‐Cercel A, Preotescu L, Asselah T, et al. Silen‐C2: early antiviral activity and safety of BI 201335 combined with peginterferon alfa‐2A and ribavirin (PegIfn/Rbv) in chronic HCV genotype‐1 patients with non‐response to PegIfn/Rbv. Journal of Hepatology 2010;52(Suppl S1):S462‐3.
-
- Sulkowski MS, Bourliere M, Bronowicki JP, Asselah T, Pawlotsky JM, Shafran SD, et al. Faldaprevir combined with peginterferon alfa‐2a and ribavirin in chronic hepatitis C virus genotype‐1 patients with prior nonresponse: SILEN‐C2 trial. Hepatology 2013;57(6):2155‐63. - PubMed
-
- Sulkowski MS, Bourliere M, Bronowicki JP, Streinu‐Cercel A, Preotescu L, Asselah T, et al. Silen‐C2: sustained virologic response (SVR) and safety of BI201335 combined with peginterferon alfa‐2A and ribavirin (P/R) in chronic HCV genotype‐1 patients with non‐response to P/R. Journal of Hepatology 2011;54(Suppl S1):S30.
Sulkowski 2014 {published data only}
-
- Sulkowski MS, Gardiner DF, Rodriguez‐Torres M, Reddy KR, Hassanein T, Jacobson I, et al. Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. New England Journal of Medicine 2014;370(3):211‐21. - PubMed
Zeuzem 2012 {published data only}
-
- Zeuzem S, Soriano V, Asselah T, Bronowicki JP, Ceausu E, Lohse AW, et al. Virologic response to an interferon‐free regimen of BI201335 and BI207127, with and without ribavirin, in treatment‐naive patients with chronic genotype‐1 HCV infection: week 12 interim results of the sound‐C2 study. Hepatology 2011;54(4 Suppl):1436A.
-
- Zeuzem S, Soriano V, Asselah T, Bronowicki JP, Lohse A, Mullhaupt B, et al. SVR4 and SVR12 with an interferon‐free regimen of BI201335 and BI207127, +/‐ribavirin, in treatment‐naive patients with chronic genotype‐1 HCV infection: interim results of sound‐C2. Journal of Hepatology 2012;56(Suppl S2):S45.
Zeuzem 2013 {published data only}
-
- Zeuzem S, Asselah T, Angus P, Zarski JP, Larrey D, Muellhaupt B, et al. Faldaprevir (BI201335), deleobuvir (BI207127) and ribavirin oral therapy for treatment‐naive HCV genotype 1: SOUND‐C1 final results. Antiviral Therapy 2013;18(8):1015‐9. - PubMed
-
- Zeuzem S, Asselah T, Angus PW, Zarski JP, Larrey DG, Mullhaupt B, et al. High sustained virologic response following interferon‐free treatment of chronic HCV GT1 infection for 4 weeks with HCV protease inhibitor BI201335, polymerase inhibitor BI207127 and ribavirin, followed by BI201335 and pegifn/ribavirin the sound‐C1 study. Hepatology 2011;54(S1):486A‐7A.
Zeuzem 2014b {published data only}
-
- Younossi ZM, Stepanova M, Zeuzem S, Dusheiko G, Esteban R, Hezode C, et al. Patient‐reported outcomes assessment in chronic hepatitis C treated with sofosbuvir and ribavirin: The VALENCE study. Journal of Hepatology 2014;61(2):228‐34. - PubMed
-
- Zeuzem S, Dusheiko GM, Salupere R, Mangia A, Flisiak R, Hyland RH, et al. Sofosbuvir + ribavirin for 12 or 24 weeks for patients with HCV genotype 2 or 3: the VALENCE trial. Hepatology 2013;58(Suppl S1):733A‐4A.
-
- Zeuzem S, Dusheiko GM, Salupere R, Mangia A, Flisiak R, Hyland RH, et al. Sofosbuvir and ribavirin in HCV genotypes 2 and 3. New England Journal of Medicine 2014;370(21):1993‐2001. - PubMed
References to ongoing studies
Izumi 2012 {published data only}
-
- Izumi N, LaTaillade M, Chayama K, Toyota J, Mochida S, Kawada N, et al. First report of peginterferon lambda/ribavirin in combination with either daclatasvir or asunaprevir in HCV genotype 1 Japanese subjects: early sustained virologic response (SVR4) results from the D‐LITE Japanese sub‐study. Hepatology 2012;56(S1):310A.
Lawitz 2014b {published data only}
-
- Lawitz E, Rodriguez‐Torres M, Nguyen T, Sheikh A, Tobias H, Galati J, et al. A phase II study of samatasvir (IDX719) in combination with simeprevir and ribavirin in treatment‐naive HCV‐infected subjects with genotypes 1B and 4 (helix‐1 study). Journal of Hepatology 2014;60(1 Suppl S):S495‐6.
Additional references
Afdhal 2014
-
- Afdhal N, Zeuzem S, Kwo P, Chojkier M, Gitlin N, Puoti M, et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. New England Journal of Medicine 2014;370(20):1889‐98. - PubMed
Bacon 2011
Balshem 2011
-
- Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401‐6. [PUBMED: 21208779] - PubMed
Basile 2014
Berger 2012
Brok 2009
Brok 2010
CDCP 1998
-
- Centers for Disease Control and Prevention. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV‐related chronic disease. MMWR. Recommendations and Reports: Morbidity and Mortality Weekly Report 1998;47(RR‐19):1‐39. - PubMed
Chan 2013
Childs‐Kean 2015
-
- Childs‐Kean LM, Hand EO. Simeprevir and sofosbuvir for treatment of chronic hepatitis C infection. Clinical Therapeutics 2015;37(2):243‐67. - PubMed
Choo 1989
-
- Choo QL, Kuo G, Weiner AJ, Overby LR, Bradley DW, Houghton M. Isolation of a cDNA clone derived from a blood‐borne non‐A, non‐B viral hepatitis genome. Science 1989;244(4902):359‐62. - PubMed
Ciani 2017
-
- Ciani O, Buyse M, Drummond M, Rasi G, Saad ED, Taylor RS. Time to review the role of surrogate end points in health policy: state of the art and the way forward. Value Health 2017;20(3):487‐95. - PubMed
Clark 2012
Coco 2014
-
- Coco B, Caraceni P, Aghemo A, Bitetto D, Bruno R, Ciancio A, et al. Triple therapy with first‐generation protease inhibitors for patients with genotype 1 chronic hepatitis C: recommendations of the Italian association for the study of the liver (AISF). Digestive and Liver Disease 2014;46(1):18‐24. - PubMed
Conteduca 2014
-
- Conteduca V, Sansonno D, Russi S, Pavone F, Dammacco F. Therapy of chronic hepatitis C virus infection in the era of direct‐acting and host‐targeting antiviral agents. Journal of Infection 2014;68(1):1‐20. - PubMed
Conti 2016
-
- Conti F, Buonfiglioli F, Scuteri A, Crespi C, Bolondi L, Caraceni P, et al. Early occurrence and recurrence of hepatocellular carcinoma in HCV‐related cirrhosis treated with direct‐acting antivirals. Journal of Hepatology 2016;65(4):727‐33. - PubMed
Deeks 2011
-
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Dubuisson 2014
-
- Dubuisson J, Cosset FL. Virology and cell biology of the hepatitis C virus life cycle: an update. Journal of Hepatology 2014;61(1 Suppl):S3‐S13. - PubMed
EASL 2015
-
- European Association for Study of Liver. EASL Recommendations on Treatment of Hepatitis C. Journal of Hepatology 2015;63(1):199‐236. - PubMed
Egger 1997
El‐Serag 2016
Elbaz 2015
Ermis 2015
FiercePharma 2017
-
- Engeneering & Biotechnology News. FiercePharma, annual Reports, company websites, SEC Filings and press releases 2017.
Flemming 1996
-
- Fleming TR, DeMets DL. Surrogate end points in clinical trials: Are we being misled?. Annals of Internal Medicine 1996, 125(7):605‐613.. - PubMed
Flemming 2012
Garattini 2016
-
- Garattini S, Jakobsen JC, Wetterslev J, Bertele V, Banzi R, Rath A, et al. Evidence‐based clinical practice: overview of threats to the validity of evidence and how to minimise them. European Journal of Internal Medicine 2016;32:13‐21. - PubMed
Gluud 2007
-
- Gluud C, Brok J, Gong Y, Koretz RL. Hepatology may have problems with putative surrogate outcome measures. Journal of Hepatology 2007;46(4):734‐42. - PubMed
Gluud 2015
-
- Gluud C, Nikolova D, Klingenberg SL. Cochrane Hepato‐Biliary Group. About Cochrane (Cochrane Review Groups (CRGs)) 2016, Issue 2. Art. No.: LIVER Vol. http://hbg.cochrane.org.
Gowan 2014
-
- Gower E, Estes C, Blach S, Razavi‐Shearer K, Razavi H. Global epidemiology and genotype distribution of the hepatitis C virus infection. Journal of Hepatology 2014;61(1):45‐57. - PubMed
Guyatt 2011a
-
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction‐GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [PUBMED: 21195583] - PubMed
Guyatt 2011b
-
- Guyatt GH, Oxman AD, Kunz R, Atkins D, Brozek J, Vist G, et al. GRADE guidelines: 2. Framing the question and deciding on important outcomes. Journal of Clinical Epidemiology 2011;64(4):395‐400. [PUBMED: 21194891] - PubMed
Guyatt 2011c
-
- Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso‐Coello P, et al. GRADE guidelines: 4. Rating the quality of evidence‐‐study limitations (risk of bias). Journal of Clinical Epidemiology 2011;64(4):407‐15. [PUBMED: 21247734] - PubMed
Guyatt 2011d
-
- Guyatt GH, Oxman AD, Montori V, Vist G, Kunz R, Brozek J, et al. GRADE guidelines: 5. Rating the quality of evidence‐‐publication bias. Journal of Clinical Epidemiology 2011;64(12):1277‐82. [PUBMED: 21802904] - PubMed
Guyatt 2011e
-
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso‐Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence‐‐imprecision. Journal of Clinical Epidemiology 2011;64(12):1283‐93. [PUBMED: 21839614] - PubMed
Guyatt 2011f
-
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 7. Rating the quality of evidence‐‐inconsistency. Journal of Clinical Epidemiology 2011;64(12):1294‐302. [PUBMED: 21803546] - PubMed
Guyatt 2011g
-
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 8. Rating the quality of evidence‐‐indirectness. Journal of Clinical Epidemiology 2011;64(12):1303‐10. [PUBMED: 21802903] - PubMed
Guyatt 2011h
-
- Guyatt GH, Oxman AD, Sultan S, Glasziou P, Akl EA, Alonso‐Coello P, et al. GRADE guidelines: 9. Rating up the quality of evidence. Journal of Clinical Epidemiology 2011;64(12):1311‐6. [PUBMED: 21802902] - PubMed
Guyatt 2013a
-
- Guyatt G, Oxman AD, Sultan S, Brozek J, Glasziou P, Alonso‐Coello P, et al. GRADE guidelines: 11. Making an overall rating of confidence in effect estimates for a single outcome and for all outcomes. Journal of Clinical Epidemiology 2013;66(2):151‐7. [PUBMED: 22542023] - PubMed
Guyatt 2013b
-
- Guyatt GH, Oxman AD, Santesso N, Helfand M, Vist G, Kunz R, et al. GRADE guidelines: 12. Preparing summary of findings tables‐binary outcomes. Journal of Clinical Epidemiology 2013;66(2):158‐72. [PUBMED: 22609141] - PubMed
Guyatt 2013c
-
- Guyatt GH, Thorlund K, Oxman AD, Walter SD, Patrick D, Furukawa TA, et al. GRADE guidelines: 13. Preparing summary of findings tables and evidence profiles‐continuous outcomes. Journal of Clinical Epidemiology 2013;66(2):173‐83. [PUBMED: 23116689] - PubMed
Götte 2016
-
- Götte M, Feld JJ. Direct‐acting antiviral agents for hepatitis C: structural and mechanistic insights. Nature Reviews. Gastroenterology & Hepatology 2016;13:338–51. - PubMed
Harbord 2006
-
- Harbord RM, Egger M, Sterne JA. A modified test for small‐study effects in meta‐analyses of controlled trials with binary endpoints. Statistics in Medicine 2006;25(20):3443‐57. - PubMed
Hauser 2014
Higgins 2003
Higgins 2011a
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.Cochrane.org.
Higgins 2011b
-
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011c
-
- Higgins JPT, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
ICH‐GCP 1997
-
- International Conference on Harmonisation Expert Working Group. International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. ICH harmonised tripartite guideline. Guideline for good clinical practice CFR & ICH Guidelines. Vol. 1, Philadelphia (PA): Barnett International/PAREXEL, 1997.
Jacobson 2011
-
- Jacobson IM, McHutchison JG, Dusheiko G, Bisceglie AM, Reddy KR, Bzowej NH, et al. Telaprevir for previously untreated chronic hepatitis C virus infection. New England Journal of Medicine 2011;364(25):2405‐16. - PubMed
Jakobsen 2014a
Jakobsen 2014b
Jakobsen 2016a
-
- Jakobsen JC, Wetterslev J, Lange T, Gluud C. Viewpoint: taking into account risks of random errors when analysing multiple outcomes in systematic reviews [editorial]. Cochrane Database of Systematic Reviews 2016, issue 3:10.1002/14651858.ED000111. [DOI: 10.1002/14651858.ED000111] - DOI - PMC - PubMed
Jakobsen 2016b
-
- Jakobsen JC, Nielsen EE, Feinberg J, Fobian K, Katakam KK, Hauser G, et al. Direct‐acting antivirals for chronic hepatitis C. Cochrane Database of Systematic Reviews 2016, Issue 4. [DOI: 10.1002/14651858.CD012143] - DOI
Kemp 2017
Kjaergard 2001
-
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. - PubMed
Koretz 2013
Koretz 2015
-
- Koretz RL, Lin KW, Ioannidis JP, Lenzer J. Is widespread screening for hepatitis C justified?. BMJ (Clinical Research Ed.) 2015;350:g7809. - PubMed
Koretz 2016
-
- Koretz, R. Direct‐acting antivirals: do they work beyond sustained virological response in chronic hepatitis C patients? [Conference proceeding at the International Liver Congress 2016, Barcelona]. EASL. 2016; Vol. ilc‐congress.eu/wp‐content/uploads/2016/programme_book/EASL_ILC%20scientific....
Lang 2013
-
- Lang D, Liang HJ, Li D, Wei X, Ma L, Jia Z. The efficacy and safety of telaprevir‐based regimens for treating chronic hepatitis C virus genotype 1 infection: a meta‐analysis of randomized trials. Internal Medicine 2013;52(6):653‐60. - PubMed
Lawitz 2013
-
- Lawitz E, Mangia A, Wyles D, Rodriguez‐Torres M, Hassanein T, Gordon SC, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. New England Journal of Medicine 2013;368(20):1878‐87. - PubMed
Lundh 2012
Manns 2006
MCDC 2015
Messina 2015
Moher 1998
-
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352(9128):609‐13. - PubMed
Moher 2009
Mok 2005
Mustafa 2013
-
- Mustafa RA, Santesso N, Brozek J, Akl EA, Walter SD, Norman G, et al. The GRADE approach is reproducible in assessing the quality of evidence of quantitative evidence syntheses. Journal of Clinical Epidemiology 2013;66(7):736‐42; quiz 742.e1‐5. [PUBMED: 23623694] - PubMed
NICE 2015a
-
- NICE. Interferon alfa (pegylated and non‐pegylated) and ribavirin for the treatment of chronic hepatitis C. www.nice.org.uk/guidance/ta75NICE.org 2015 (accessed 2 March 2016).
NICE 2015b
-
- NICE. Sofosbuvir for treating chronic hepatitis C. www.nice.org.uk/guidance/ta330/chapter/2‐The‐technology 2015 (accessed 2 March 2016).
Pockros 2015
-
- Pockros PJ. Direct‐acting antivirals for the treatment of hepatitis C virus infection. www.uptodate.com/contents/direct‐acting‐antivirals‐for‐the‐treatment‐of‐... 2015 (accessed 2 March 2016).
Poordad 2011
Poordad 2012
-
- Poordad F, Dieterich D. Treating hepatitis C: current standard of care and emerging direct‐acting antiviral agents. Journal of Viral Hepatitis 2012;19(7):449‐64. - PubMed
Reig 2016
-
- Reig M, Marino Z, Perello C, Inarrairaegui M, Ribeiro A, Lens S, et al. Unexpected high rate of early tumor recurrence in patients with HCV‐related HCC undergoing interferon‐free therapy. J Hepatol. 2016;65(4):719‐26.. - PubMed
Reig 2017
-
- Reig M, Boix L, Marino Z, Torres F, Forns X, Bruix J. Liver Cancer Emergence Associated with Antiviral Treatment: An Immune Surveillance Failure?. Semin Liver Dis. 2017;37(2):109‐18.. - PubMed
RevMan 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Righi 2015
Roche 2015
Savović 2012a
-
- Savović J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Annals of Internal Medicine 2012;157(6):429‐38. - PubMed
Savović 2012b
-
- Savović J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Health Technology Assessment 2012;16(35):1‐82. - PubMed
Scheel 2013
Schultz 2010
-
- Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Annals of Internal Medicine 2010;152(11):726‐32. - PubMed
Schulz 1995
-
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. Journal of the American Medical Association 1995;273(5):408‐12. - PubMed
Schünemann 2011
-
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and ‘Summary of findings' tables. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Smith‐Palmer 2015
Spradling 2012
-
- Spradling PR, Rupp L, Moorman AC, Lu M, Teshale EH, Gordon SC, et al. Chronic Hepatitis Cohort Study Investigators. Hepatitis B and C virus infection among 1.2 million persons with access to care: factors associated with testing and infection prevalence. Clinical Infectious Diseases 2012;55(8):1047‐55. - PMC - PubMed
Sterne 2011
-
- Sterne JAC, Egger M, Moher D (editors). Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Intervention. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Sweeting 2004
-
- Sweeting MJ, Sutton AJ, Lambert PC. What to add to nothing? Use and avoidance of continuity corrections in meta‐analysis of sparse data. Statistics in Medicine 2004;23(9):1351‐75. - PubMed
Thein 2008
-
- Thein HH, Yi Q, Dore GJ, Krahn MD. Estimation of stage‐specific fibrosis progression rates in chronic hepatitis C virus infection: a meta‐analysis and meta‐regression.. Hepatology 2008;48(2):418. - PubMed
Thomas 2009
Thorlund 2011
-
- Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User manual for Trial Sequential Analysis (TSA). ctu.dk/tsa/files/tsa_manual.pdf 2011 (accessed 2 March 2016).
TSA 2011 [Computer program]
-
- Copenhagen Trial Unit. TSA ‐ Trial Sequential Analysis. Version 0.9 Beta. Copenhagen: Copenhagen Trial Unit, 2011.
Wandeler 2015
-
- Wandeler G, Dufour JF, Bruggmann P, Rauch A. Hepatitis C: a changing epidemic. Swiss Medical Weekly 2015;145:w14093. - PubMed
Wang 2017
-
- Wang C, Ji D, Chen J, Shao Q, Li B, Liu J, Wu V, Wong A, Wang Y, Zhang X, Lu L, Wong C. Tsang S, Zhanf Z, Sun J, Hou J, Chen G, Lau G. Hepatitis due to reactivation of hepatitis B virus in endemic areas among patients with hepatitis C treated with direct‐acting antiviral agents. Clin Gastroenterol Hepatol 2017; 15:132‐6. - PubMed
Wetterslev 2008
-
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial Sequential Analysis may establish when firm evidence is reached in cumulative meta‐analysis. Journal of Clinical Epidemiology 2008;61(1):64‐75. - PubMed
Wetterslev 2009
WHO 2014
-
- WHO. Hepatitis C Fact sheet N°164. www.who.int/mediacentre/factsheets/fs164/en/ 2015 (accessed 2 March 2016).
Wood 2008
Wyles 2015
-
- Wyles DL, Ruane PJ, Sulkowski MS, Dieterich D, Luetkemeyer A, Morgan TR, et al. Daclatasvir plus sofosbuvir for HCV in patients coinfected with HIV‐1. New England Journal of Medicine 2015;373(8):714‐25. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

