Targeting the Myofibroblastic Cancer-Associated Fibroblast Phenotype Through Inhibition of NOX4
- PMID: 28922779
- PMCID: PMC5903651
- DOI: 10.1093/jnci/djx121
Targeting the Myofibroblastic Cancer-Associated Fibroblast Phenotype Through Inhibition of NOX4
Abstract
Background: Cancer-associated fibroblasts (CAFs) are tumor-promoting and correlate with poor survival in many cancers, which has led to their emergence as potential therapeutic targets. However, effective methods to manipulate these cells clinically have yet to be developed.
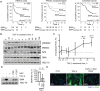
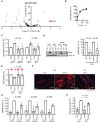
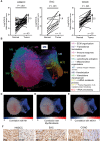
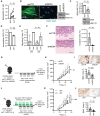
Methods: CAF accumulation and prognostic significance in head and neck cancer (oral, n = 260; oropharyngeal, n = 271), and colorectal cancer (n = 56) was analyzed using immunohistochemistry. Mechanisms regulating fibroblast-to-myofibroblast transdifferentiation were investigated in vitro using RNA interference/pharmacological inhibitors followed by polymerase chain reaction (PCR), immunoblotting, immunofluorescence, and functional assays. RNA sequencing/bioinformatics and immunohistochemistry were used to analyze NAD(P)H Oxidase-4 (NOX4) expression in different human tumors. NOX4's role in CAF-mediated tumor progression was assessed in vitro, using CAFs from multiple tissues in Transwell and organotypic culture assays, and in vivo, using xenograft (n = 9-15 per group) and isograft (n = 6 per group) tumor models. All statistical tests were two-sided.
Results: Patients with moderate/high levels of myofibroblastic-CAF had a statistically significant decrease in cancer-specific survival rates in each cancer type analyzed (hazard ratios [HRs] = 1.69-7.25, 95% confidence intervals [CIs] = 1.11 to 31.30, log-rank P ≤ .01). Fibroblast-to-myofibroblast transdifferentiation was dependent on a delayed phase of intracellular reactive oxygen species, generated by NOX4, across different anatomical sites and differentiation stimuli. A statistically significant upregulation of NOX4 expression was found in multiple human cancers (P < .001), strongly correlating with myofibroblastic-CAFs (r = 0.65-0.91, adjusted P < .001). Genetic/pharmacological inhibition of NOX4 was found to revert the myofibroblastic-CAF phenotype ex vivo (54.3% decrease in α-smooth muscle actin [α-SMA], 95% CI = 10.6% to 80.9%, P = .009), prevent myofibroblastic-CAF accumulation in vivo (53.2%-79.0% decrease in α-SMA across different models, P ≤ .02) and slow tumor growth (30.6%-64.0% decrease across different models, P ≤ .04).
Conclusions: These data suggest that pharmacological inhibition of NOX4 may have broad applicability for stromal targeting across cancer types.
© The Author 2017. Published by Oxford University Press.
Figures
Comment in
-
Targeted Therapy for Cancer-Associated Fibroblasts: Are We There Yet?J Natl Cancer Inst. 2018 Jan 1;110(1). doi: 10.1093/jnci/djx131. J Natl Cancer Inst. 2018. PMID: 28922783 No abstract available.
References
-
- Hanahan D, Weinberg RA.. Hallmarks of cancer: The next generation. Cell. 2011;144(5):646–674. - PubMed
-
- Erez N, Truitt M, Olson P, Arron ST, Hanahan D.. Cancer-associated fibroblasts are activated in incipient neoplasia to orchestrate tumor-promoting inflammation in an NF-kappaB-dependent manner. Cancer Cell. 2010;17(2):135–147. - PubMed
-
- Kraman M, Bambrough PJ, Arnold JN, et al.Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein-alpha. Science. 2010;330(6005):827–830. - PubMed
-
- Marsh D, Suchak K, Moutasim KA, et al.Stromal features are predictive of disease mortality in oral cancer patients. J Pathol. 2011;223(4):470–481. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

