The Transcription Factors COUP-TFI and COUP-TFII have Distinct Roles in Arealisation and GABAergic Interneuron Specification in the Early Human Fetal Telencephalon
- PMID: 28922831
- PMCID: PMC5903418
- DOI: 10.1093/cercor/bhx185
The Transcription Factors COUP-TFI and COUP-TFII have Distinct Roles in Arealisation and GABAergic Interneuron Specification in the Early Human Fetal Telencephalon
Abstract
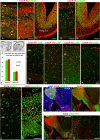
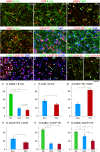
In human telencephalon at 8-12 postconceptional weeks, ribonucleic acid quantitative sequencing and immunohistochemistry revealed cortical chicken ovalbumin upstream promotor-transcription factor 1 (COUP-TFI) expression in a high ventro-posterior to low anterior gradient except for raised immunoreactivity in the anterior ventral pallium. Unlike in mouse, COUP-TFI and SP8 were extensively co-expressed in dorsal sensory neocortex and dorsal hippocampus whereas COUPTFI/COUPTFII co-expression defined ventral temporal cortex and ventral hippocampus. In the ganglionic eminences (GEs) COUP-TFI immunoreactivity demarcated the proliferative zones of caudal GE (CGE), dorsal medial GE (MGE), MGE/lateral GE (LGE) boundary, and ventral LGE whereas COUP-TFII was limited to ventral CGE and the MGE/LGE boundary. Co-labeling with gamma amino butyric acidergic interneuron markers revealed that COUP-TFI was expressed in subpopulations of either MGE-derived (SOX6+) or CGE-derived (calretinin+/SP8+) interneurons. COUP-TFII was mainly confined to CGE-derived interneurons. Twice as many GAD67+ cortical cells co-labeled for COUP-TFI than for COUP-TFII. A fifth of COUP-TFI cells also co-expressed COUP-TFII, and cells expressing either transcription factor followed posterior or anterio-lateral pathways into the cortex, therefore, a segregation of migration pathways according to COUP-TF expression as proposed in mouse was not observed. In cultures differentiated from isolated human cortical progenitors, many cells expressed either COUP-TF and 30% also co-expressed GABA, however no cells expressed NKX2.1. This suggests interneurons could be generated intracortically from progenitors expressing either COUP-TF.
Keywords: SP8; cerebral cortex development; ganglionic eminences; hippocampus development; interneuron migration; ventral pallium.
© The Author 2017. Published by Oxford University Press.
Figures





References
-
- Alfano C, Magrinelli E, Harb K, Hevner RF, Studer M. 2014. a. Postmitotic control of sensory area specification during neocortical development. Nature Commun. 5:5632. - PubMed
-
- Alfano C, Viola L, Heng JIT, Pirozzi M, Clarkson M, Flore G, De Maio A, Schedl A, Guillemot F, Studer M. 2011. COUP-TFI promotes radial migration and proper morphology of callosal projection neurons by repressing Rnd2 expression. Development. 138:4685–4697. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

