Bioengineered Submucosal Organoids for In Vitro Modeling of Colorectal Cancer
- PMID: 28922975
- PMCID: PMC5653148
- DOI: 10.1089/ten.tea.2017.0397
Bioengineered Submucosal Organoids for In Vitro Modeling of Colorectal Cancer
Abstract
The physical nature of the tumor microenvironment significantly impacts tumor growth, invasion, and response to drugs. Most in vitro tumor models are designed to study the effects of extracellular matrix (ECM) stiffness on tumor cells, while not addressing the effects of ECM's specific topography. In this study, we bioengineered submucosal organoids, using primary smooth muscle cells embedded in collagen I hydrogel, which produce aligned and parallel fiber topography similar to those found in vivo. The fiber organization in the submucosal organoids induced an epithelial phenotype in spheroids of colorectal carcinoma cells (HCT-116), which were embedded within the organoids. Conversely, unorganized fibers drove a mesenchymal phenotype in the tumor cells. HCT-116 cells in organoids with aligned fibers showed no WNT signaling activation, and conversely, WNT signaling activation was observed in organoids with disrupted fibers. Consequently, HCT-116 cells in the aligned condition exhibited decreased cellular proliferation and reduced sensitivity to 5-fluorouracil chemotherapeutic treatment compared to cells in the unorganized construct. Collectively, the results establish a unique colorectal tumor organoid model to study the effects of stromal topography on cancer cell phenotype, proliferation, and ultimately, chemotherapeutic susceptibility. In the future, such organoids can utilize patient-derived cells for precision medicine applications.
Keywords: collagen organization; colorectal cancer; epithelial-to-mesenchymal transition; extracellular matrix; tumor models.
Conflict of interest statement
No competing financial interests exist.
Figures
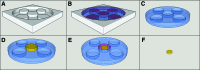

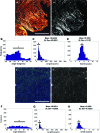

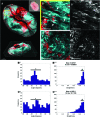
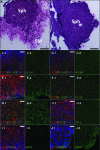

References
-
- Luca A.C., Mersch S., Deenen R., Schmidt S., Messner I., Schäfer K.L., Baldus S.E., Huckenbeck W., Piekorz R.P., Knoefel W.T., Krieg A., and Stoecklein N.H. Impact of the 3D microenvironment on phenotype, gene expression, and EGFR inhibition of colorectal cancer cell lines. PLoS One 8, e59689, 2013 - PMC - PubMed
-
- Catalano V., Turdo A., Di Franco S., Dieli F., Todaro M., and Stassi G. Tumor and its microenvironment: a synergistic interplay. Semin Cancer Biol 23, 522, 2013 - PubMed
-
- Pietras K., and Östman A. Hallmarks of cancer: interactions with the tumor stroma. Exp Cell Res 316, 1324, 2010 - PubMed
-
- Cichon M.A., Radisky E.S., and Radisky D.C. Identifying the stroma as a critical player in radiation-induced mammary tumor development. Cancer Cell 19, 571, 2011 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

