Regulatory networks underlying mycorrhizal development delineated by genome-wide expression profiling and functional analysis of the transcription factor repertoire of the plant symbiotic fungus Laccaria bicolor
- PMID: 28923004
- PMCID: PMC5604158
- DOI: 10.1186/s12864-017-4114-7
Regulatory networks underlying mycorrhizal development delineated by genome-wide expression profiling and functional analysis of the transcription factor repertoire of the plant symbiotic fungus Laccaria bicolor
Abstract
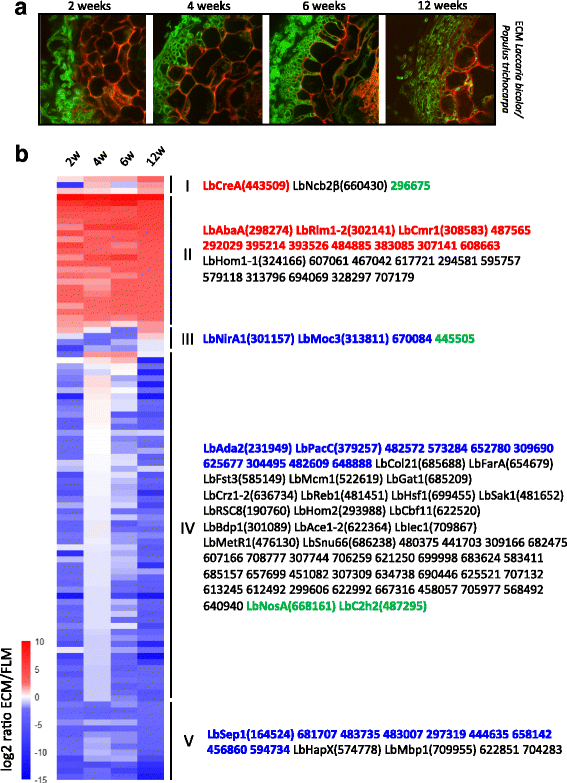
Background: Ectomycorrhizal (ECM) fungi develop a mutualistic symbiotic interaction with the roots of their host plants. During this process, they undergo a series of developmental transitions from the running hyphae in the rhizosphere to the coenocytic hyphae forming finger-like structures within the root apoplastic space. These transitions, which involve profound, symbiosis-associated metabolic changes, also entail a substantial transcriptome reprogramming with coordinated waves of differentially expressed genes. To date, little is known about the key transcriptional regulators driving these changes, and the aim of the present study was to delineate and functionally characterize the transcription factor (TF) repertoire of the model ECM fungus Laccaria bicolor.
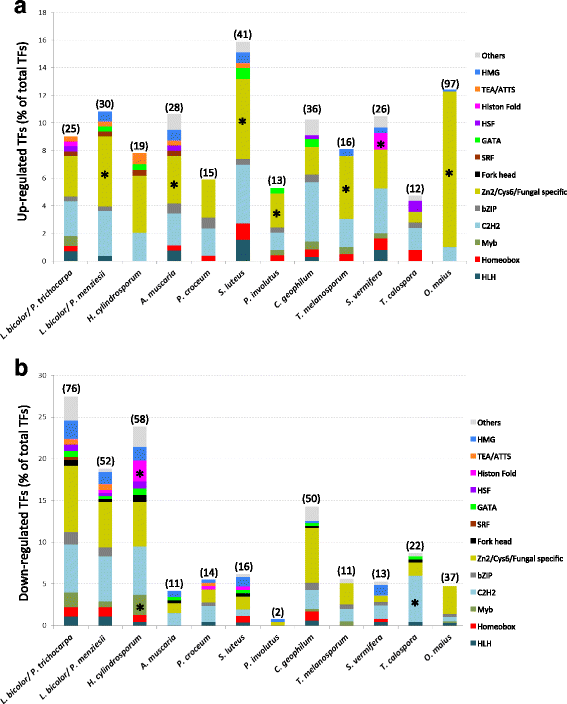
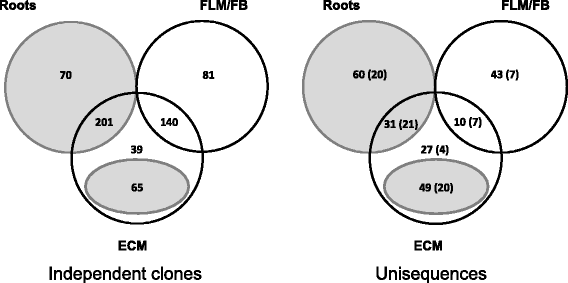
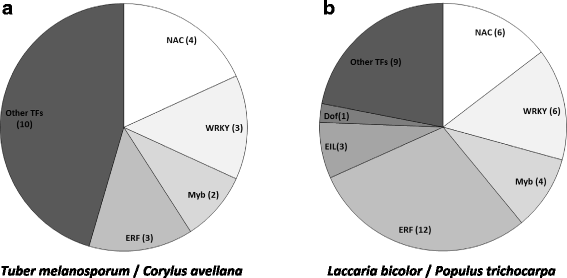
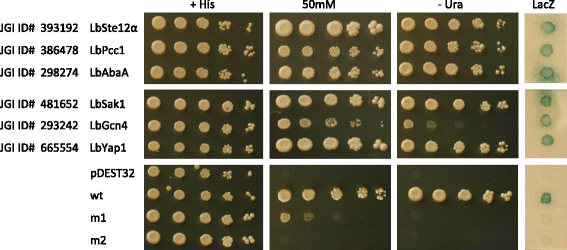
Results: We curated the L. bicolor gene models coding for transcription factors and assessed their expression and regulation in Poplar and Douglas fir ectomycorrhizae. We identified 285 TFs, 191 of which share a significant similarity with known transcriptional regulators. Expression profiling of the corresponding transcripts identified TF-encoding fungal genes differentially expressed in the ECM root tips of both host plants. The L. bicolor core set of differentially expressed TFs consists of 12 and 22 genes that are, respectively, upregulated and downregulated in symbiotic tissues. These TFs resemble known fungal regulators involved in the control of fungal invasive growth, fungal cell wall integrity, carbon and nitrogen metabolism, invasive stress response and fruiting-body development. However, this core set of mycorrhiza-regulated TFs seems to be characteristic of L. bicolor and our data suggest that each mycorrhizal fungus has evolved its own set of ECM development regulators. A subset of the above TFs was functionally validated with the use of a heterologous, transcription activation assay in yeast, which also allowed the identification of previously unknown, transcriptionally active yet secreted polypeptides designated as Secreted Transcriptional Activator Proteins (STAPs).
Conclusions: Transcriptional regulators required for ECM symbiosis development in L. bicolor have been uncovered and classified through genome-wide analysis. This study also identifies the STAPs as a new class of potential ECM effectors, highly expressed in mycorrhizae, which may be involved in the control of the symbiotic root transcriptome.
Keywords: Transcription factors; ectomycorrhiza development; secreted proteins; symbiosis; transcriptional activator trap assay; transcriptome; yeast.
Conflict of interest statement
Ethics approval and consent to participate
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

