A novel method for extracting nucleic acids from dried blood spots for ultrasensitive detection of low-density Plasmodium falciparum and Plasmodium vivax infections
- PMID: 28923054
- PMCID: PMC5604154
- DOI: 10.1186/s12936-017-2025-3
A novel method for extracting nucleic acids from dried blood spots for ultrasensitive detection of low-density Plasmodium falciparum and Plasmodium vivax infections
Abstract
Background: Greater Mekong Subregion countries are committed to eliminating Plasmodium falciparum malaria by 2025. Current elimination interventions target infections at parasite densities that can be detected by standard microscopy or rapid diagnostic tests (RDTs). More sensitive detection methods have been developed to detect lower density "asymptomatic" infections that may represent an important transmission reservoir. These ultrasensitive polymerase chain reaction (usPCR) tests have been used to identify target populations for mass drug administration (MDA). To date, malaria usPCR tests have used either venous or capillary blood sampling, which entails complex sample collection, processing and shipping requirements. An ultrasensitive method performed on standard dried blood spots (DBS) would greatly facilitate the molecular surveillance studies needed for targeting elimination interventions.
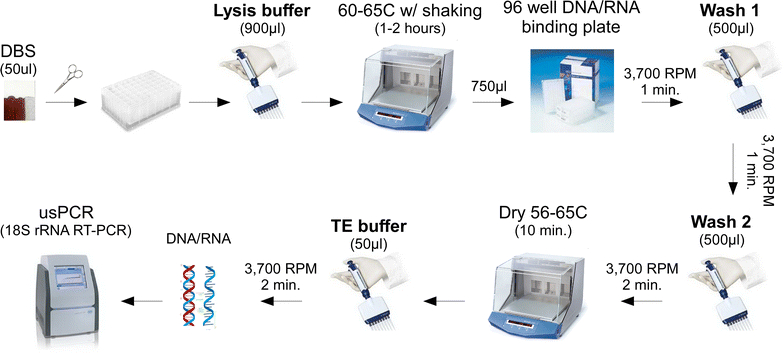
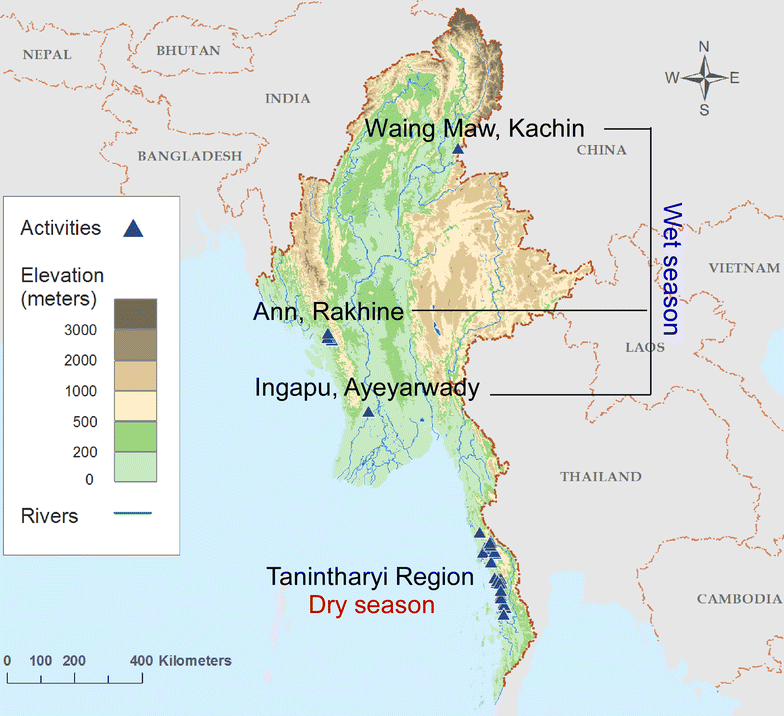
Methods: A highly sensitive method for detecting Plasmodium falciparum and P. vivax 18S ribosomal RNA from DBS was developed by empirically optimizing nucleic acid extraction conditions. The limit of detection (LoD) was determined using spiked DBS samples that were dried and stored under simulated field conditions. Further, to assess its utility for routine molecular surveillance, two cross-sectional surveys were performed in Myanmar during the wet and dry seasons.
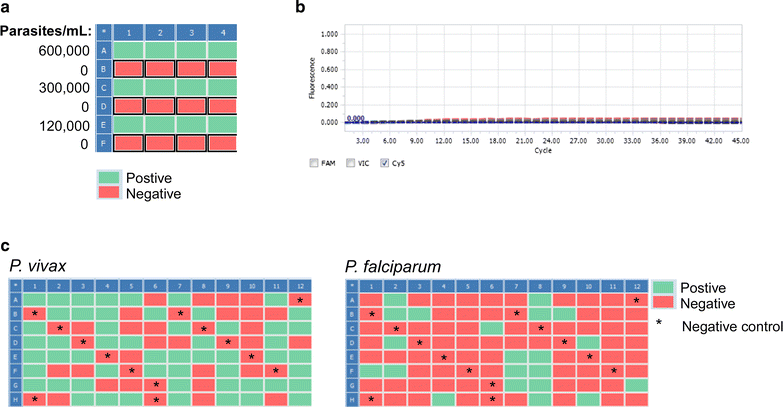
Results: The lower LoD of the DBS-based ultrasensitive assay was 20 parasites/mL for DBS collected on Whatman 3MM filter paper and 23 parasites/mL for Whatman 903 Protein Saver cards-equivalent to 1 parasite per 50 µL DBS. This is about 5000-fold more sensitive than standard RDTs and similar to the LoD of ≤16-22 parasites/mL reported for other ultrasensitive methods based on whole blood. In two cross-sectional surveys in Myanmar, nearly identical prevalence estimates were obtained from contemporaneous DBS samples and capillary blood samples collected during the wet and dry season.
Conclusions: The DBS-based ultrasensitive method described in this study shows equal sensitivity as previously described methods based on whole blood, both in its limit of detection and prevalence estimates in two field surveys. The reduced cost and complexity of this method will allow for the scale-up of surveillance studies to target MDA and other malaria elimination interventions, and help lead to a better understanding of the epidemiology of low-density malaria infections.
Keywords: Asymptomatic infection; DBS; Diagnostics; Dried blood spot; Limits of detection; Low transmission; Malaria; Malaria elimination; Molecular surveillance; Myanmar; Plasmodium falciparum; Plasmodium vivax; Southeast Asia; Ultrasensitive PCR.
Figures

Similar articles
-
An ultrasensitive reverse transcription polymerase chain reaction assay to detect asymptomatic low-density Plasmodium falciparum and Plasmodium vivax infections in small volume blood samples.Malar J. 2015 Dec 23;14:520. doi: 10.1186/s12936-015-1038-z. Malar J. 2015. PMID: 26701778 Free PMC article.
-
Geographical heterogeneity in prevalence of subclinical malaria infections at sentinel endemic sites of Myanmar.Parasit Vectors. 2019 Feb 18;12(1):83. doi: 10.1186/s13071-019-3330-1. Parasit Vectors. 2019. PMID: 30777127 Free PMC article.
-
Microscopic and molecular evidence of the presence of asymptomatic Plasmodium falciparum and Plasmodium vivax infections in an area with low, seasonal and unstable malaria transmission in Ethiopia.BMC Infect Dis. 2015 Aug 5;15:310. doi: 10.1186/s12879-015-1070-1. BMC Infect Dis. 2015. PMID: 26242405 Free PMC article.
-
Ultrasensitive Diagnostics for Low-Density Asymptomatic Plasmodium falciparum Infections in Low-Transmission Settings.J Clin Microbiol. 2021 Mar 19;59(4):e01508-20. doi: 10.1128/JCM.01508-20. Print 2021 Mar 19. J Clin Microbiol. 2021. PMID: 33148707 Free PMC article. Review.
-
Comparative performance of PCR using DNA extracted from dried blood spots and whole blood samples for malaria diagnosis: a meta-analysis.Sci Rep. 2021 Mar 1;11(1):4845. doi: 10.1038/s41598-021-83977-5. Sci Rep. 2021. PMID: 33649410 Free PMC article.
Cited by
-
Molecular malaria surveillance using a novel protocol for extraction and analysis of nucleic acids retained on used rapid diagnostic tests.Sci Rep. 2020 Jul 23;10(1):12305. doi: 10.1038/s41598-020-69268-5. Sci Rep. 2020. PMID: 32703999 Free PMC article.
-
Prevalence of molecular markers of sulfadoxine-pyrimethamine and artemisinin resistance in Plasmodium falciparum from Pakistan.Malar J. 2018 Dec 17;17(1):471. doi: 10.1186/s12936-018-2620-y. Malar J. 2018. PMID: 30558587 Free PMC article.
-
High throughput sequencing of T-cell receptor repertoire using dry blood spots.J Transl Med. 2019 Feb 18;17(1):47. doi: 10.1186/s12967-019-1796-4. J Transl Med. 2019. PMID: 30777078 Free PMC article.
-
Improved limit of detection for zoonotic Plasmodium knowlesi and P. cynomolgi surveillance using reverse transcription for total nucleic acid preserved samples or dried blood spots.PLoS Negl Trop Dis. 2025 Feb 6;19(2):e0012129. doi: 10.1371/journal.pntd.0012129. eCollection 2025 Feb. PLoS Negl Trop Dis. 2025. PMID: 39913530 Free PMC article.
-
An improved nucleic acid extraction method from dried blood spots for amplification of Plasmodium falciparum kelch13 for detection of artemisinin resistance.Malar J. 2019 Jun 11;18(1):192. doi: 10.1186/s12936-019-2817-8. Malar J. 2019. PMID: 31185976 Free PMC article.
References
-
- Leang R, Taylor WR, Bouth DM, Song L, Tarning J, Char MC, et al. Evidence of Plasmodium falciparum malaria multidrug resistance to artemisinin and piperaquine in western Cambodia: dihydroartemisinin-piperaquine open-label multicenter clinical assessment. Antimicrob Agents Chemother. 2015;59:4719–4726. doi: 10.1128/AAC.00835-15. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

