Patient-reported outcomes of baricitinib in patients with rheumatoid arthritis and no or limited prior disease-modifying antirheumatic drug treatment
- PMID: 28923098
- PMCID: PMC5604362
- DOI: 10.1186/s13075-017-1410-1
Patient-reported outcomes of baricitinib in patients with rheumatoid arthritis and no or limited prior disease-modifying antirheumatic drug treatment
Abstract
Background: This study evaluates patient-reported outcomes (PROs) in a double-blind, phase III study of baricitinib as monotherapy or combined with methotrexate (MTX) in patients with active rheumatoid arthritis (RA) with no or minimal prior conventional synthetic disease-modifying antirheumatic drugs (DMARDs) and naïve to biological DMARDs.
Methods: Patients were randomized 4:3:4 to MTX administered once weekly (N = 210), baricitinib monotherapy (4 mg once daily (QD), N = 159), or combination of baricitinib (4 mg QD) and MTX (baricitinib + MTX, N = 215). PROs included the Patient's Global Assessment of Disease Activity (PtGA), patient's assessment of pain, Health Assessment Questionnaire-Disability Index (HAQ-DI), Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F), duration of morning joint stiffness (MJS), worst joint pain, worst tiredness, Work Productivity and Activity Impairment-Rheumatoid Arthritis (WPAI-RA), Short Form 36 version 2, Acute (SF-36); and EuroQol 5-Dimensions (EQ-5D) Health State Profile. Comparisons were assessed with analysis of covariance (ANCOVA) and logistic regression models.
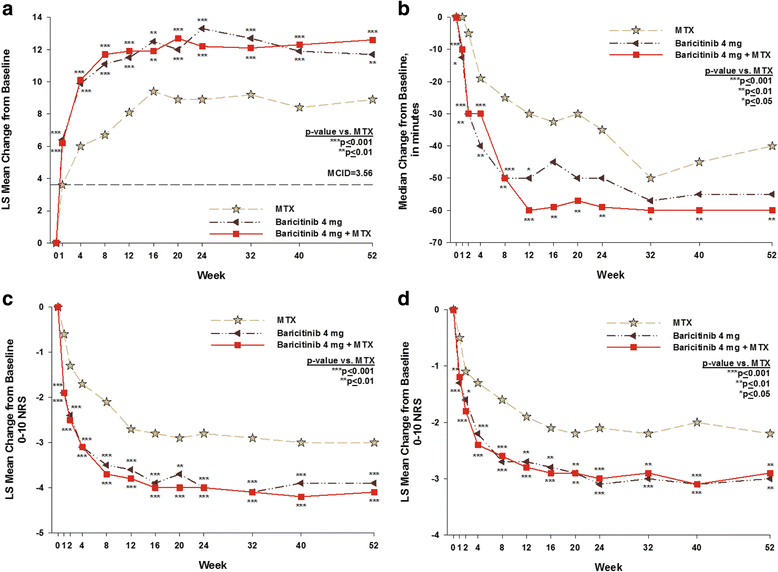
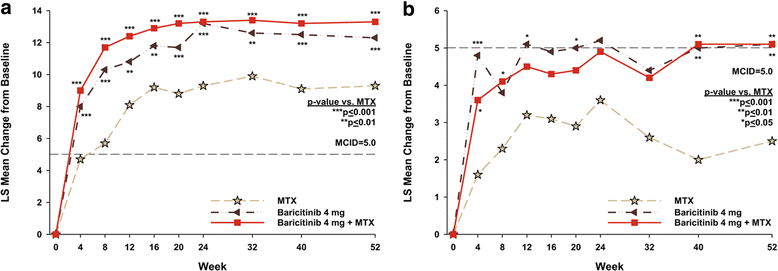
Results: Compared to MTX, patients in both baricitinib groups reported greater improvement (p ≤ 0.01) in HAQ-DI, PtGA, pain, fatigue, worst join pain, SF-36 physical component score, and EQ-5D at weeks 24 and 52. For the SF-36 mental component score, patients in both baricitinib groups reported statistically significant improvements (p ≤ 0.01) at week 52 compared to MTX-treated patients. Statistically significant improvements (p ≤ 0.05) were observed with the WPAI-RA for the baricitinib groups vs. MTX at week 24 and for the WPAI-RA daily activity and work productivity measures for baricitinib + MTX at week 52.
Conclusions: In this study, baricitinib alone or in combination with MTX, when used as initial therapy, resulted in significant improvement compared to MTX in the majority of the pre-specified PRO measures.
Trial registration: ClinicalTrials.gov, NCT01711359 . Registered on 18 October 2012.
Keywords: Baricitinib; HAQ-DI; Health-related quality of life; JAK inhibitor; PRO; RA; Rheumatoid; health status indicators; tsDMARD.
Conflict of interest statement
Ethics approval and consent to participate
The study was conducted in accordance with ethical principles of the Declaration of Helsinki and Good Clinical Practice guidelines and was approved by each center’s institutional review board or ethics committee. All patients provided written informed consent.
Consent for publication
Not applicable.
Competing interests
Financial Support and Disclosures: MS has received consulting fees from AbbVie, Amgen, Bristol-Myers Squibb, Eli Lilly and Company, Johnson & Johnson, Novartis, Roche, and UCB (< US$10,000 each) and speaking fees from AbbVie and Bristol-Myers Squibb (> US$10,000). TT has received consulting fees from Pfizer Japan Inc., Astra Zeneca K.K., Eli Lilly Japan K.K., Novartis Pharma K.K., Daiichi Sankyo Co., Ltd., Nipponkayaku Co., Ltd, Janssen Pharmaceutical K.K., Merck Serono Co., Ltd., Takeda Pharmaceutical Co., Ltd. (< US$10,000 each), and from Mitsubishi Tanabe Pharma Co., Astellas Pharma Inc, AbbVie GK, Bristol–Myers K.K. Asahi Kasei Medical K.K (> US$10,000 each), and speaking fees from Celtrion, Nipponkayaku Co., Ltd, Pfizer Japan Inc., UCB Japan, Diaichi Sankyo Co., Ltd., Takeda Pharmaceutical Co., Ltd., (< US$10,000 each) and from Chugai Pharmaceutical Co,. Ltd., AbbVie GK., Bristol–Myers K.K., Eisai Co., Ltd., Mitsubishi Tanabe Pharma Co., Janssen Pharmaceutical K.K., and Astellas Pharma Inc., (> US$10,000 each). RF has received consulting fees from AbbVie, Amgen, Bristol–Myers Squibb, Janssen, Eli Lilly and Company, and Sanofi-Aventis (< US$10,000 each), and consulting fees from Pfizer and UCB (> US$10,000 each). CG, AD, DS, W-LK, J-EW, TC, and TR are employees of Eli Lilly and Company and all, except TC and AD are shareholders. PD has received consulting fees from Eli Lilly and Company. SS has received consulting fees from Abbvie, Eli Lilly, Roche, and UCB. RPH has no conflicts of interest related to this manuscript. RvV has received consulting fees from AbbVie, Biotest, BMS, Celgene, Crescendo, GSK, Janssen, Lilly, Merck, Novartis, Pfizer, Roche, UCB, and Vertex and research grants from AbbVie, Amgen, BMS, GSK, Pfizer, Roche, and UCB. CAFZ has received consulting fees from Merck, Pfizer, Sanofi, and Eli Lilly and Company (< US$10,000 each).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Fleischmann R, Schiff M, van der Heijde D, Ramos-Remus C, Spindler A, Stanislav M, Zerbini CAF, Gurbuz S, Dickson C, de Bono S, et al. Baricitinib, methotrexate, or combination in patients with rheumatoid arthritis and no or limited prior disease-modifying antirheumatic drug treatment. Arthritis Rheumatol. 2017;69:506–17. doi:10.1002/art.39953. - PMC - PubMed
-
- Bruce B, Fries JF. The Health Assessment Questionnaire (HAQ) Clin Exp Rheumatol. 2005;23:S14–8. - PubMed
-
- Ramey D, Fries J, Singh G. The Health Assessment Questionnaire 1995 — status and review. In: Spilker B, editor. Quality of life and pharmacoeconomics in clinical trials. 2. Philadelphia: Lippincott-Raven; 1996. pp. 227–37.
-
- Felson DT, Anderson JJ, Boers M, Bombardier C, Chernoff M, Fried B, et al. The American College of Rheumatology preliminary core set of disease activity measures for rheumatoid arthritis clinical trials. The Committee on Outcome Measures in Rheumatoid Arthritis Clinical Trials. Arthritis Rheum. 1993;36:729–40. doi: 10.1002/art.1780360601. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

