Dose Comparison Study of Allogeneic Mesenchymal Stem Cells in Patients With Ischemic Cardiomyopathy (The TRIDENT Study)
- PMID: 28923793
- PMCID: PMC8742223
- DOI: 10.1161/CIRCRESAHA.117.311827
Dose Comparison Study of Allogeneic Mesenchymal Stem Cells in Patients With Ischemic Cardiomyopathy (The TRIDENT Study)
Abstract
Rationale: Cell dose and concentration play crucial roles in phenotypic responses to cell-based therapy for heart failure.
Objective: To compare the safety and efficacy of 2 doses of allogeneic bone marrow-derived human mesenchymal stem cells identically delivered in patients with ischemic cardiomyopathy.
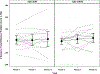
Methods and results: Thirty patients with ischemic cardiomyopathy received in a blinded manner either 20 million (n=15) or 100 million (n=15) allogeneic human mesenchymal stem cells via transendocardial injection (0.5 cc per injection × 10 injections per patient). Patients were followed for 12 months for safety and efficacy end points. There were no treatment-emergent serious adverse events at 30 days or treatment-related serious adverse events at 12 months. The Major Adverse Cardiac Event rate was 20.0% (95% confidence interval [CI], 6.9% to 50.0%) in 20 million and 13.3% (95% CI, 3.5% to 43.6%) in 100 million (P=0.58). Worsening heart failure rehospitalization was 20.0% (95% CI, 6.9% to 50.0%) in 20 million and 7.1% (95% CI, 1.0% to 40.9%) in 100 million (P=0.27). Whereas scar size reduced to a similar degree in both groups: 20 million by -6.4 g (interquartile range, -13.5 to -3.4 g; P=0.001) and 100 million by -6.1 g (interquartile range, -8.1 to -4.6 g; P=0.0002), the ejection fraction improved only with 100 million by 3.7 U (interquartile range, 1.1 to 6.1; P=0.04). New York Heart Association class improved at 12 months in 35.7% (95% CI, 12.7% to 64.9%) in 20 million and 42.9% (95% CI, 17.7% to 71.1%) in 100 million. Importantly, proBNP (pro-brain natriuretic peptide) increased at 12 months in 20 million by 0.32 log pg/mL (95% CI, 0.02 to 0.62; P=0.039), but not in 100 million (-0.07 log pg/mL; 95% CI, -0.36 to 0.23; P=0.65; between group P=0.07).
Conclusions: Although both cell doses reduced scar size, only the 100 million dose increased ejection fraction. This study highlights the crucial role of cell dose in the responses to cell therapy. Determining optimal dose and delivery is essential to advance the field, decipher mechanism(s) of action and enhance planning of pivotal Phase III trials.
Clinical trial registration: URL: http://www.clinicaltrials.gov. Unique identifier: NCT02013674.
Keywords: bone marrow; cell-based therapy; heart failure; hospitalization; stem cells.
© 2017 American Heart Association, Inc.
Figures











Comment in
-
Posology for Regenerative Therapy.Circ Res. 2017 Nov 10;121(11):1213-1215. doi: 10.1161/CIRCRESAHA.117.312074. Circ Res. 2017. PMID: 29122941 Free PMC article. No abstract available.
References
-
- de la Fuente LM, Stertzer SH, Argentieri J, Penaloza E, Miano J, Koziner B, Bilos C, Altman PA. Transendocardial autologous bone marrow in chronic myocardial infarction using a helical needle catheter: 1-year follow-up in an open-label, nonrandomized, single-center pilot study (the tabmmi study). American heart journal. 2007;154:79 e71–77 - PubMed
-
- Perin EC, Willerson JT, Pepine CJ, Henry TD, Ellis SG, Zhao DX, Silva GV, Lai D, Thomas JD, Kronenberg MW, Martin AD, Anderson RD, Traverse JH, Penn MS, Anwaruddin S, Hatzopoulos AK, Gee AP, Taylor DA, Cogle CR, Smith D, Westbrook L, Chen J, Handberg E, Olson RE, Geither C, Bowman S, Francescon J, Baraniuk S, Piller LB, Simpson LM, Loghin C, Aguilar D, Richman S, Zierold C, Bettencourt J, Sayre SL, Vojvodic RW, Skarlatos SI, Gordon DJ, Ebert RF, Kwak M, Moye LA, Simari RD, Cardiovascular Cell Therapy Research N. Effect of transendocardial delivery of autologous bone marrow mononuclear cells on functional capacity, left ventricular function, and perfusion in chronic heart failure: The focus-cctrn trial. JAMA : the journal of the American Medical Association. 2012;307:1717–1726 - PMC - PubMed
-
- Williams AR, Trachtenberg B, Velazquez DL, McNiece I, Altman P, Rouy D, Mendizabal AM, Pattany PM, Lopera GA, Fishman J, Zambrano JP, Heldman AW, Hare JM. Intramyocardial stem cell injection in patients with ischemic cardiomyopathy: Functional recovery and reverse remodeling. Circulation research. 2011;108:792–796 - PMC - PubMed
-
- Losordo DW, Henry TD, Davidson C, Sup Lee J, Costa MA, Bass T, Mendelsohn F, Fortuin FD, Pepine CJ, Traverse JH, Amrani D, Ewenstein BM, Riedel N, Story K, Barker K, Povsic TJ, Harrington RA, Schatz RA, Investigators AC. Intramyocardial, autologous cd34+ cell therapy for refractory angina. Circulation research. 2011;109:428–436 - PMC - PubMed
-
- Hare JM, Fishman JE, Gerstenblith G, DiFede Velazquez DL, Zambrano JP, Suncion VY, Tracy M, Ghersin E, Johnston PV, Brinker JA, Breton E, Davis-Sproul J, Schulman IH, Byrnes J, Mendizabal AM, Lowery MH, Rouy D, Altman P, Wong Po Foo C, Ruiz P, Amador A, Da Silva J, McNiece IK, Heldman AW, George R, Lardo A. Comparison of allogeneic vs autologous bone marrow-derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: The poseidon randomized trial. JAMA : the journal of the American Medical Association. 2012;308:2369–2379 - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

