Antiviral Activity of Bictegravir and Cabotegravir against Integrase Inhibitor-Resistant SIVmac239 and HIV-1
- PMID: 28923862
- PMCID: PMC5700299
- DOI: 10.1128/AAC.01695-17
Antiviral Activity of Bictegravir and Cabotegravir against Integrase Inhibitor-Resistant SIVmac239 and HIV-1
Abstract
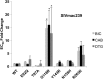
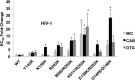
Animal models are essential to study novel antiretroviral drugs, resistance-associated mutations (RAMs), and treatment strategies. Bictegravir (BIC) is a novel potent integrase strand transfer inhibitor (INSTI) that has shown promising results against HIV-1 infection in vitro and in vivo and against clinical isolates with resistance against INSTIs. BIC has a higher genetic barrier to the development of resistance than two clinically approved INSTIs, termed raltegravir and elvitegravir. Another clinically approved INSTI, dolutegravir (DTG) also possesses a high genetic barrier to resistance, while a fourth compound, termed cabotegravir (CAB), is currently in late phases of clinical development. Here we report the susceptibilities of simian immunodeficiency virus (SIV) and HIV-1 integrase (IN) mutants containing various RAMs to BIC, CAB, and DTG. BIC potently inhibited SIV and HIV-1 in single cycle infection with 50% effective concentrations (EC50s) in the low nM range. In single cycle SIV infections, none of the E92Q, T97A, Y143R, or N155H substitutions had a significant effect on susceptibility to BIC (≤4-fold increase in EC50), whereas G118R and R263K conferred ∼14-fold and ∼6-fold increases in EC50, respectively. In both single and multiple rounds of HIV-1 infections, BIC remained active against the Y143R, N155H, R263K, R263K/M50I, and R263K/E138K mutants (≤4-fold increase in EC50). In multiple rounds of infection, the G140S/Q148H combination of substitutions decreased HIV-1 susceptibility to BIC 4.8-fold compared to 16.8- and 7.4-fold for CAB and DTG, respectively. BIC possesses an excellent resistance profile in regard to HIV and SIV and could be useful in nonhuman primate models of HIV infection.
Keywords: HIV; SIV; bictegravir; cabotegravir; dolutegravir; integrase; integrase strand transfer inhibitors; resistance.
Copyright © 2017 American Society for Microbiology.
Figures
References
-
- Tobin NH, Learn GH, Holte SE, Wang Y, Melvin AJ, McKernan JL, Pawluk DM, Mohan KM, Lewis PF, Mullins JI, Frenkel LM. 2005. Evidence that low-level viremias during effective highly active antiretroviral therapy result from two processes: expression of archival virus and replication of virus. J Virol 79:9625–9634. doi: 10.1128/JVI.79.15.9625-9634.2005. - DOI - PMC - PubMed
-
- Bailey JR, Sedaghat AR, Kieffer T, Brennan T, Lee PK, Wind-Rotolo M, Haggerty CM, Kamireddi AR, Liu Y, Lee J, Persaud D, Gallant JE, Cofrancesco J Jr, Quinn TC, Wilke CO, Ray SC, Siliciano JD, Nettles RE, Siliciano RF. 2006. Residual human immunodeficiency virus type 1 viremia in some patients on antiretroviral therapy is dominated by a small number of invariant clones rarely found in circulating CD4+ T cells. J Virol 80:6441–6457. doi: 10.1128/JVI.00591-06. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

