H-IPSE Is a Pathogen-Secreted Host Nucleus-Infiltrating Protein (Infiltrin) Expressed Exclusively by the Schistosoma haematobium Egg Stage
- PMID: 28923894
- PMCID: PMC5695104
- DOI: 10.1128/IAI.00301-17
H-IPSE Is a Pathogen-Secreted Host Nucleus-Infiltrating Protein (Infiltrin) Expressed Exclusively by the Schistosoma haematobium Egg Stage
Abstract
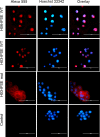
Urogenital schistosomiasis, caused by the parasitic trematode Schistosoma haematobium, affects over 112 million people worldwide. As with Schistosoma mansoni infections, the pathology of urogenital schistosomiasis is related mainly to the egg stage, which induces granulomatous inflammation of affected tissues. Schistosoma eggs and their secretions have been studied extensively for the related organism S. mansoni, which is more amenable to laboratory studies. Indeed, we have shown that IPSE/alpha-1 (here M-IPSE), a major protein secreted from S. mansoni eggs, can infiltrate host cells. Although the function of M-IPSE is unknown, its ability to translocate to the nuclei of host cells and bind DNA suggests a possible role in immune modulation of host cell tissues. Whether IPSE homologs are expressed in other schistosome species has not been investigated. Here, we describe the cloning of two paralog genes, H03-IPSE and H06-IPSE, which are orthologs of M-IPSE, from egg cDNA of S. haematobium Using PCR and immunodetection, we confirmed that the expression of these genes is restricted to the egg stage and female adult worms, while the H-IPSE protein is detectable only in mature eggs and not adults. We show that both H03-IPSE and H06-IPSE proteins can infiltrate HTB-9 bladder cells when added exogenously to culture medium. Monopartite C-terminal nuclear localization sequence (NLS) motifs conserved in H03-IPSE, SKRRRKY, and H06-IPSE SKRGRKY, are responsible for targeting the proteins to the nucleus of HTB-9 cells, as demonstrated by site-directed mutagenesis and green fluorescent protein (GFP) tagging. Thus, S. haematobium eggs express IPSE homologs that appear to perform similar functions in infiltrating host cells.
Keywords: Schistosoma haematobium; nuclear localization signal; schistosomiasis.
Copyright © 2017 Pennington et al.
Figures





References
-
- Fitzsimmons CM, Schramm G, Jones FM, Chalmers IW, Hoffmann KF, Grevelding CG, Wuhrer M, Hokke CH, Haas H, Doenhoff MJ, Dunne DW. 2005. Molecular characterization of omega-1: a hepatotoxic ribonuclease from Schistosoma mansoni eggs. Mol Biochem Parasitol 144:123–127. doi:10.1016/j.molbiopara.2005.08.003. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

