Exceptionally tight membrane-binding may explain the key role of the synaptotagmin-7 C2A domain in asynchronous neurotransmitter release
- PMID: 28923929
- PMCID: PMC5635908
- DOI: 10.1073/pnas.1710708114
Exceptionally tight membrane-binding may explain the key role of the synaptotagmin-7 C2A domain in asynchronous neurotransmitter release
Abstract
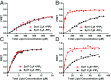
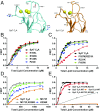
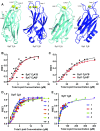
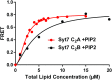
Synaptotagmins (Syts) act as Ca2+ sensors in neurotransmitter release by virtue of Ca2+-binding to their two C2 domains, but their mechanisms of action remain unclear. Puzzlingly, Ca2+-binding to the C2B domain appears to dominate Syt1 function in synchronous release, whereas Ca2+-binding to the C2A domain mediates Syt7 function in asynchronous release. Here we show that crystal structures of the Syt7 C2A domain and C2AB region, and analyses of intrinsic Ca2+-binding to the Syt7 C2 domains using isothermal titration calorimetry, did not reveal major differences that could explain functional differentiation between Syt7 and Syt1. However, using liposome titrations under Ca2+ saturating conditions, we show that the Syt7 C2A domain has a very high membrane affinity and dominates phospholipid binding to Syt7 in the presence or absence of l-α-phosphatidylinositol 4,5-diphosphate (PIP2). For Syt1, the two Ca2+-saturated C2 domains have similar affinities for membranes lacking PIP2, but the C2B domain dominates binding to PIP2-containing membranes. Mutagenesis revealed that the dramatic differences in membrane affinity between the Syt1 and Syt7 C2A domains arise in part from apparently conservative residue substitutions, showing how striking biochemical and functional differences can result from the cumulative effects of subtle residue substitutions. Viewed together, our results suggest that membrane affinity may be a key determinant of the functions of Syt C2 domains in neurotransmitter release.
Keywords: X-ray crystallography; membrane binding; neurotransmitter release; synaptotagmin-1; synaptotagmin-7.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


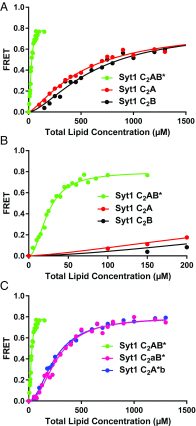
References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

