Endothelial insulin receptors differentially control insulin signaling kinetics in peripheral tissues and brain of mice
- PMID: 28923931
- PMCID: PMC5635907
- DOI: 10.1073/pnas.1710625114
Endothelial insulin receptors differentially control insulin signaling kinetics in peripheral tissues and brain of mice
Abstract
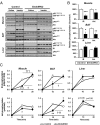
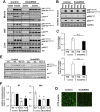
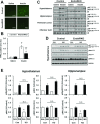
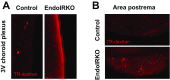
Insulin receptors (IRs) on endothelial cells may have a role in the regulation of transport of circulating insulin to its target tissues; however, how this impacts on insulin action in vivo is unclear. Using mice with endothelial-specific inactivation of the IR gene (EndoIRKO), we find that in response to systemic insulin stimulation, loss of endothelial IRs caused delayed onset of insulin signaling in skeletal muscle, brown fat, hypothalamus, hippocampus, and prefrontal cortex but not in liver or olfactory bulb. At the level of the brain, the delay of insulin signaling was associated with decreased levels of hypothalamic proopiomelanocortin, leading to increased food intake and obesity accompanied with hyperinsulinemia and hyperleptinemia. The loss of endothelial IRs also resulted in a delay in the acute hypoglycemic effect of systemic insulin administration and impaired glucose tolerance. In high-fat diet-treated mice, knockout of the endothelial IRs accelerated development of systemic insulin resistance but not food intake and obesity. Thus, IRs on endothelial cells have an important role in transendothelial insulin delivery in vivo which differentially regulates the kinetics of insulin signaling and insulin action in peripheral target tissues and different brain regions. Loss of this function predisposes animals to systemic insulin resistance, overeating, and obesity.
Keywords: brain insulin action; endothelial cells; feeding behavior; insulin receptor; insulin resistance.
Conflict of interest statement
Conflict of interest statement: Masahiro Konishi is an employee of Daiichi Sankyo and was on sabbatical leave at Joslin Diabetes Center when the study was completed.
Figures









References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

