Direct engagement of the PI3K pathway by mutant KIT dominates oncogenic signaling in gastrointestinal stromal tumor
- PMID: 28923937
- PMCID: PMC5635919
- DOI: 10.1073/pnas.1711449114
Direct engagement of the PI3K pathway by mutant KIT dominates oncogenic signaling in gastrointestinal stromal tumor
Abstract
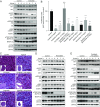
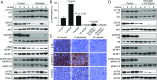
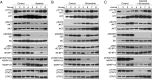
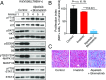
Gastrointestinal stromal tumors (GISTs) predominantly harbor activating mutations in the receptor tyrosine kinase KIT. To genetically dissect in vivo the requirement of different signal transduction pathways emanating from KIT for tumorigenesis, the oncogenic KitV558Δ mutation was combined with point mutations abrogating specific phosphorylation sites on KIT. Compared with single-mutant KitV558Δ/+ mice, double-mutant KitV558Δ;Y567F/Y567F knock-in mice lacking the SRC family kinase-binding site on KIT (pY567) exhibited attenuated MAPK signaling and tumor growth. Surprisingly, abrogation of the PI3K-binding site (pY719) in KitV558Δ;Y719F/Y719F mice prevented GIST development, although the interstitial cells of Cajal (ICC), the cells of origin of GIST, were normal. Pharmacologic inhibition of the PI3K pathway in tumor-bearing KitV558Δ/+ mice with the dual PI3K/mTOR inhibitor voxtalisib, the pan-PI3K inhibitor pilaralisib, and the PI3K-alpha-restricted inhibitor alpelisib each diminished tumor proliferation. The addition of the MEK inhibitor PD-325901 or binimetinib further decreased downstream KIT signaling. Moreover, combining PI3K and MEK inhibition was effective against imatinib-resistant KitV558Δ;T669I/+ tumors.
Keywords: GIST; Kit; PI3K; mouse.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Hirota S, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577–580. - PubMed
-
- Huizinga JD, et al. W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature. 1995;373:347–349. - PubMed
-
- Maeda H, et al. Requirement of c-kit for development of intestinal pacemaker system. Development. 1992;116:369–375. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

