Defective decidualization during and after severe preeclampsia reveals a possible maternal contribution to the etiology
- PMID: 28923940
- PMCID: PMC5635883
- DOI: 10.1073/pnas.1706546114
Defective decidualization during and after severe preeclampsia reveals a possible maternal contribution to the etiology
Abstract
In preeclampsia (PE), cytotrophoblast (CTB) invasion of the uterus and spiral arteries is often shallow. Thus, the placenta's role has been a focus. In this study, we tested the hypothesis that decidual defects are an important determinant of the placental phenotype. We isolated human endometrial stromal cells from nonpregnant donors with a previous pregnancy that was complicated by severe PE (sPE). Compared with control cells, they failed to decidualize in vitro as demonstrated by morphological criteria and the analysis of stage-specific antigens (i.e., IGFBP1, PRL). These results were bolstered by global transcriptional profiling data that showed they were transcriptionally inert. Additionally, we used laser microdissection to isolate the decidua from tissue sections of the maternal-fetal interface in sPE. Global transcriptional profiling revealed defects in gene expression. Also, decidual cells from patients with sPE, which dedifferentiated in vitro, failed to redecidualize in culture. Conditioned medium from these cells failed to support CTB invasion. To mimic aspects of the uterine environment in normal pregnancy, we added PRL and IGFBP1, which enhanced invasion. These data suggested that failed decidualization is an important contributor to down-regulated CTB invasion in sPE. Future studies will be aimed at determining whether this discovery has translational potential with regard to assessing a woman's risk of developing this pregnancy complication.
Keywords: cytotrophoblast; decidua; human endometrial stromal cells; preeclampsia; transcriptomics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
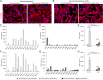



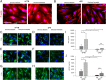
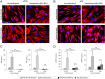
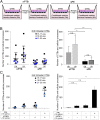
Comment in
-
Transcriptional signature of the decidua in preeclampsia.Proc Natl Acad Sci U S A. 2018 Jun 12;115(24):E5434-E5436. doi: 10.1073/pnas.1802234115. Epub 2018 May 30. Proc Natl Acad Sci U S A. 2018. PMID: 29848633 Free PMC article. No abstract available.
-
Reply to Liu et al.: Decidualization defect in severe preeclampsia.Proc Natl Acad Sci U S A. 2018 Aug 14;115(33):E7656-E7657. doi: 10.1073/pnas.1802916115. Epub 2018 Aug 1. Proc Natl Acad Sci U S A. 2018. PMID: 30068606 Free PMC article. No abstract available.
References
-
- Roberts JM, Cooper DW. Pathogenesis and genetics of pre-eclampsia. Lancet. 2001;357:53–56. - PubMed
-
- American College of Obstetricians and Gynecologists; Task Force on Hypertension in Pregnancy Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122:1122–1131. - PubMed
-
- Brosens IA, Robertson WB, Dixon HG. The role of the spiral arteries in the pathogenesis of preeclampsia. Obstet Gynecol Annu. 1972;1:177–191. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

