Cross-scale effects of neural interactions during human neocortical seizure activity
- PMID: 28923948
- PMCID: PMC5635869
- DOI: 10.1073/pnas.1702490114
Cross-scale effects of neural interactions during human neocortical seizure activity
Erratum in
-
Correction for Eissa et al., Cross-scale effects of neural interactions during human neocortical seizure activity.Proc Natl Acad Sci U S A. 2017 Oct 24;114(43):E9182. doi: 10.1073/pnas.1717066114. Epub 2017 Oct 16. Proc Natl Acad Sci U S A. 2017. PMID: 29073116 Free PMC article. No abstract available.
Abstract
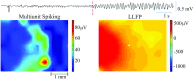
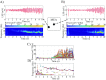
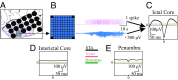
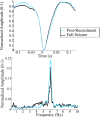
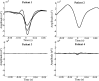
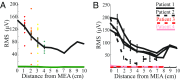
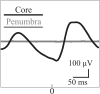
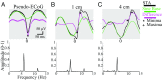
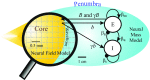
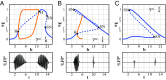
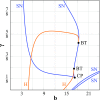
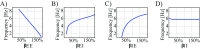
Small-scale neuronal networks may impose widespread effects on large network dynamics. To unravel this relationship, we analyzed eight multiscale recordings of spontaneous seizures from four patients with epilepsy. During seizures, multiunit spike activity organizes into a submillimeter-sized wavefront, and this activity correlates significantly with low-frequency rhythms from electrocorticographic recordings across a 10-cm-sized neocortical network. Notably, this correlation effect is specific to the ictal wavefront and is absent interictally or from action potential activity outside the wavefront territory. To examine the multiscale interactions, we created a model using a multiscale, nonlinear system and found evidence for a dual role for feedforward inhibition in seizures: while inhibition at the wavefront fails, allowing seizure propagation, feedforward inhibition of the surrounding centimeter-scale networks is activated via long-range excitatory connections. Bifurcation analysis revealed that distinct dynamical pathways for seizure termination depend on the surrounding inhibition strength. Using our model, we found that the mesoscopic, local wavefront acts as the forcing term of the ictal process, while the macroscopic, centimeter-sized network modulates the oscillatory seizure activity.
Keywords: epilepsy; feedforward inhibition; multiscale interactions; nonlinear dynamics; seizures.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

