Leaderless secreted peptide signaling molecule alters global gene expression and increases virulence of a human bacterial pathogen
- PMID: 28923955
- PMCID: PMC5635878
- DOI: 10.1073/pnas.1705972114
Leaderless secreted peptide signaling molecule alters global gene expression and increases virulence of a human bacterial pathogen
Abstract
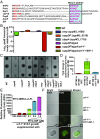
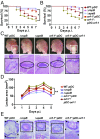
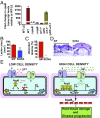
Successful pathogens use complex signaling mechanisms to monitor their environment and reprogram global gene expression during specific stages of infection. Group A Streptococcus (GAS) is a major human pathogen that causes significant disease burden worldwide. A secreted cysteine protease known as streptococcal pyrogenic exotoxin B (SpeB) is a key virulence factor that is produced abundantly during infection and is critical for GAS pathogenesis. Although identified nearly a century ago, the molecular basis for growth phase control of speB gene expression remains unknown. We have discovered that GAS uses a previously unknown peptide-mediated intercellular signaling system to control SpeB production, alter global gene expression, and enhance virulence. GAS produces an eight-amino acid leaderless peptide [SpeB-inducing peptide (SIP)] during high cell density and uses the secreted peptide for cell-to-cell signaling to induce population-wide speB expression. The SIP signaling pathway includes peptide secretion, reimportation into the cytosol, and interaction with the intracellular global gene regulator Regulator of Protease B (RopB), resulting in SIP-dependent modulation of DNA binding and regulatory activity of RopB. Notably, SIP signaling causes differential expression of ∼14% of GAS core genes. Several genes that encode toxins and other virulence genes that enhance pathogen dissemination and infection are significantly up-regulated. Using three mouse infection models, we show that the SIP signaling pathway is active during infection and contributes significantly to GAS pathogenesis at multiple host anatomic sites. Together, our results delineate the molecular mechanisms involved in a previously undescribed virulence regulatory pathway of an important human pathogen and suggest new therapeutic strategies.
Keywords: SpeB; Streptococcus pyogenes; leaderless peptide; quorum sensing; virulence regulation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

