Multiscale characterization of the mineral phase at skeletal sites of breast cancer metastasis
- PMID: 28923958
- PMCID: PMC5635895
- DOI: 10.1073/pnas.1708161114
Multiscale characterization of the mineral phase at skeletal sites of breast cancer metastasis
Abstract
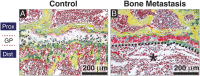
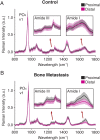
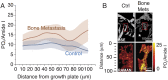
Skeletal metastases, the leading cause of death in advanced breast cancer patients, depend on tumor cell interactions with the mineralized bone extracellular matrix. Bone mineral is largely composed of hydroxyapatite (HA) nanocrystals with physicochemical properties that vary significantly by anatomical location, age, and pathology. However, it remains unclear whether bone regions typically targeted by metastatic breast cancer feature distinct HA materials properties. Here we combined high-resolution X-ray scattering analysis with large-area Raman imaging, backscattered electron microscopy, histopathology, and microcomputed tomography to characterize HA in mouse models of advanced breast cancer in relevant skeletal locations. The proximal tibial metaphysis served as a common metastatic site in our studies; we identified that in disease-free bones this skeletal region contained smaller and less-oriented HA nanocrystals relative to ones that constitute the diaphysis. We further observed that osteolytic bone metastasis led to a decrease in HA nanocrystal size and perfection in remnant metaphyseal trabecular bone. Interestingly, in a model of localized breast cancer, metaphyseal HA nanocrystals were also smaller and less perfect than in corresponding bone in disease-free controls. Collectively, these results suggest that skeletal sites prone to tumor cell dissemination contain less-mature HA (i.e., smaller, less-perfect, and less-oriented crystals) and that primary tumors can further increase HA immaturity even before secondary tumor formation, mimicking alterations present during tibial metastasis. Engineered tumor models recapitulating these spatiotemporal dynamics will permit assessing the functional relevance of the detected changes to the progression and treatment of breast cancer bone metastasis.
Keywords: Raman imaging; X-ray scattering; bone metastasis; bone mineral nanostructure; breast cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures










References
-
- Kozlow W, Guise TA. Breast cancer metastasis to bone: Mechanisms of osteolysis and implications for therapy. J Mammary Gland Biol Neoplasia. 2005;10:169–180. - PubMed
-
- Fratzl P, Gupta HS, Paschalis EP, Roschger P. Structure and mechanical quality of the collagen-mineral nano-composite in bone. J Mater Chem. 2004;14:2115–2123.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

