Small genome symbiont underlies cuticle hardness in beetles
- PMID: 28923972
- PMCID: PMC5635926
- DOI: 10.1073/pnas.1712857114
Small genome symbiont underlies cuticle hardness in beetles
Abstract
Beetles, representing the majority of the insect species diversity, are characterized by thick and hard cuticle, which plays important roles for their environmental adaptation and underpins their inordinate diversity and prosperity. Here, we report a bacterial endosymbiont extremely specialized for sustaining beetle's cuticle formation. Many weevils are associated with a γ-proteobacterial endosymbiont lineage Nardonella, whose evolutionary origin is estimated as older than 100 million years, but its functional aspect has been elusive. Sequencing of Nardonella genomes from diverse weevils unveiled drastic size reduction to 0.2 Mb, in which minimal complete gene sets for bacterial replication, transcription, and translation were present but almost all of the other metabolic pathway genes were missing. Notably, the only metabolic pathway retained in the Nardonella genomes was the tyrosine synthesis pathway, identifying tyrosine provisioning as Nardonella's sole biological role. Weevils are armored with hard cuticle, tyrosine is the principal precursor for cuticle formation, and experimental suppression of Nardonella resulted in emergence of reddish and soft weevils with low tyrosine titer, confirming the importance of Nardonella-mediated tyrosine production for host's cuticle formation and hardening. Notably, Nardonella's tyrosine synthesis pathway was incomplete, lacking the final step transaminase gene. RNA sequencing identified host's aminotransferase genes up-regulated in the bacteriome. RNA interference targeting the aminotransferase genes induced reddish and soft weevils with low tyrosine titer, verifying host's final step regulation of the tyrosine synthesis pathway. Our finding highlights an impressively intimate and focused aspect of the host-symbiont metabolic integrity via streamlined evolution for a single biological function of ecological relevance.
Keywords: Nardonella; genome; symbiont; tyrosine; weevil.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
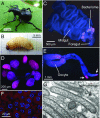


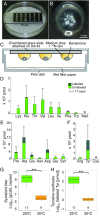
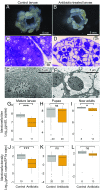
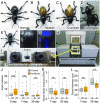


Comment in
-
Evolution: Weevils Get Tough on Symbiotic Tyrosine.Curr Biol. 2017 Dec 4;27(23):R1282-R1284. doi: 10.1016/j.cub.2017.10.031. Curr Biol. 2017. PMID: 29207272
References
-
- Buchner P. Endosymbiosis of Animals with Plant Microorganisms. Interscience; New York: 1965.
-
- Boutzis K, Miller TA. Insect Symbiosis. CRC; Boca Raton, FL: 2003.
-
- Zchori-Fein E, Miller TA. Manipulative Tenants: Bacteria Associated with Arthropods. CRC; Boca Raton, FL: 2011.
-
- Moran NA, McCutcheon JP, Nakabachi A. Genomics and evolution of heritable bacterial symbionts. Annu Rev Genet. 2008;42:165–190. - PubMed
-
- Douglas AE. The microbial dimension in insect nutritional ecology. Funct Ecol. 2009;23:38–47.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

