Flow-induced gelation of microfiber suspensions
- PMID: 28923973
- PMCID: PMC5642717
- DOI: 10.1073/pnas.1710927114
Flow-induced gelation of microfiber suspensions
Abstract
The flow behavior of fiber suspensions has been studied extensively, especially in the limit of dilute concentrations and rigid fibers; at the other extreme, however, where the suspensions are concentrated and the fibers are highly flexible, much less is understood about the flow properties. We use a microfluidic method to produce uniform concentrated suspensions of high aspect ratio, flexible microfibers, and we demonstrate the shear thickening and gelling behavior of such microfiber suspensions, which, to the best of our knowledge, has not been reported previously. By rheological means, we show that flowing the suspension triggers the irreversible formation of topological entanglements of the fibers resulting in an entangled water-filled network. This phenomenon suggests that flexible fiber suspensions can be exploited to produce a new family of flow-induced gelled materials, such as porous hydrogels. A significant consequence of these flow properties is that the microfiber suspension is injectable through a needle, from which it can be extruded directly as a hydrogel without any chemical reactions or further treatments. Additionally, we show that this fiber hydrogel is a soft, viscoelastic, yield-stress material.
Keywords: flexible fiber suspension; flow-induced gelation; hydrogel.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


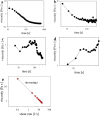

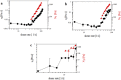


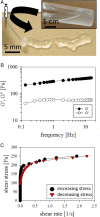
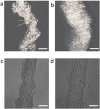




References
-
- Keshtkar M, Heuzey MC, Carreau PJ. Rheological behavior of fiber-filled model suspensions: Effect of fiber flexibility. J Rheol (NYNY) 2009;53:631–650.
-
- Zirnsak MA, Hur DU, Boger DV. Normal stresses in fibre suspensions. J Nonnewton Fluid Mech. 1994;54:153–193.
-
- Ganani E, Powell RL. Suspensions of rodlike particles: Literature review and data correlations. J Compos Mater. 1985;19:194–215.
-
- Cheng X, McCoy JH, Israelachvili JN, Cohen I. Imaging the microscopic structure of shear thinning and thickening colloidal suspensions. Science. 2011;333:1276–1279. - PubMed
-
- Wagner NJ, Brady JF. Shear thickening in colloidal dispersions. Phys Today. 2009;62:27–32.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

