Ibrutinib for chronic graft-versus-host disease after failure of prior therapy
- PMID: 28924018
- PMCID: PMC6033048
- DOI: 10.1182/blood-2017-07-793786
Ibrutinib for chronic graft-versus-host disease after failure of prior therapy
Abstract
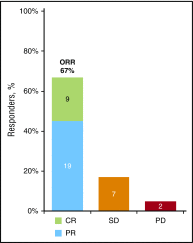
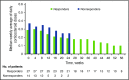
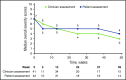
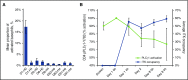
Chronic graft-versus-host disease (cGVHD) is a serious complication of allogeneic stem cell transplantation with few effective options available after failure of corticosteroids. B and T cells play a role in the pathophysiology of cGVHD. Ibrutinib inhibits Bruton tyrosine kinase in B cells and interleukin-2-inducible T-cell kinase in T cells. In preclinical models, ibrutinib reduced severity of cGVHD. This multicenter, open-label study evaluated the safety and efficacy of ibrutinib in patients with active cGVHD with inadequate response to corticosteroid-containing therapies. Forty-two patients who had failed 1 to 3 prior treatments received ibrutinib (420 mg) daily until cGVHD progression. The primary efficacy end point was cGVHD response based on 2005 National Institutes of Health criteria. At a median follow-up of 13.9 months, best overall response was 67%; 71% of responders showed a sustained response for ≥20 weeks. Responses were observed across involved organs evaluated. Most patients with multiple cGVHD organ involvement had a multiorgan response. Median corticosteroid dose in responders decreased from 0.29 mg/kg per day at baseline to 0.12 mg/kg per day at week 49; 5 responders discontinued corticosteroids. The most common adverse events were fatigue, diarrhea, muscle spasms, nausea, and bruising. Plasma levels of soluble factors associated with inflammation, fibrosis, and cGVHD significantly decreased over time with ibrutinib. Ibrutinib resulted in clinically meaningful responses with acceptable safety in patients with ≥1 prior treatments for cGVHD. Based on these results, ibrutinib was approved in the United States for treatment of adult patients with cGVHD after failure of 1 or more lines of systemic therapy. This trial was registered at www.clinicaltrials.gov as #NCT02195869.
© 2017 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure
Figures
Comment in
-
Chronic GVHD: progress in salvage treatment?Blood. 2017 Nov 23;130(21):2237-2238. doi: 10.1182/blood-2017-10-808626. Blood. 2017. PMID: 29170191 No abstract available.
References
-
- Socié G, Stone JV, Wingard JR, et al. ; Late Effects Working Committee of the International Bone Marrow Transplant Registry. Long-term survival and late deaths after allogeneic bone marrow transplantation. N Engl J Med. 1999;341(1):14-21. - PubMed
-
- Lee SJ, Klein JP, Barrett AJ, et al. . Severity of chronic graft-versus-host disease: association with treatment-related mortality and relapse. Blood. 2002;100(2):406-414. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

