A B-Z junction induced by an A … A mismatch in GAC repeats in the gene for cartilage oligomeric matrix protein promotes binding with the hZαADAR1 protein
- PMID: 28924040
- PMCID: PMC5704460
- DOI: 10.1074/jbc.M117.796235
A B-Z junction induced by an A … A mismatch in GAC repeats in the gene for cartilage oligomeric matrix protein promotes binding with the hZαADAR1 protein
Abstract
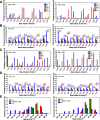
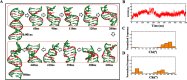
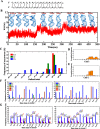
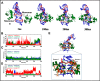
GAC repeat expansion from five to seven in the exonic region of the gene for cartilage oligomeric matrix protein (COMP) leads to pseudoachondroplasia, a skeletal abnormality. However, the molecular mechanism by which GAC expansions in the COMP gene lead to skeletal dysplasias is poorly understood. Here we used molecular dynamics simulations, which indicate that an A … A mismatch in a d(GAC)6·d(GAC)6 duplex induces negative supercoiling, leading to a local B-to-Z DNA transition. This transition facilitates the binding of d(GAC)7·d(GAC)7 with the Zα-binding domain of human adenosine deaminase acting on RNA 1 (ADAR1, hZαADAR1), as confirmed by CD, NMR, and microscale thermophoresis studies. The CD results indicated that hZαADAR1 recognizes the zigzag backbone of d(GAC)7·d(GAC)7 at the B-Z junction and subsequently converts it into Z-DNA via the so-called passive mechanism. Molecular dynamics simulations carried out for the modeled hZαADAR1-d(GAC)6d(GAC)6 complex confirmed the retention of previously reported important interactions between the two molecules. These findings suggest that hZαADAR1 binding with the GAC hairpin stem in COMP can lead to a non-genetic, RNA editing-mediated substitution in COMP that may then play a crucial role in the development of pseudoachondroplasia.
Keywords: CD; NMR; RNA editing; cartilage oligomeric matrix protein; human adar1 protein; molecular dynamics; pseudoachdroplasia; trinucleotide repeat disease.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures




References
-
- Pearson C. E., Nichol Edamura K., and Cleary J. D. (2005) Repeat instability: mechanisms of dynamic mutations. Nat. Rev. Genet. 6, 729–742 - PubMed
-
- Briggs M. D., Hoffman S. M., King L. M., Olsen A. S., Mohrenweiser H., Leroy J. G., Mortier G. R., Rimoin D. L., Lachman R. S., and Gaines E. S. (1995) Pseudoachondroplasia and multiple epiphyseal dysplasia due to mutations in the cartilage oligomeric matrix protein gene. Nat. Genet. 10, 330–336 - PubMed
-
- Délot E., King L. M., Briggs M. D., Wilcox W. R., and Cohn D. H. (1999) Trinucleotide expansion mutations in the cartilage oligomeric matrix protein (COMP) gene. Hum. Mol. Genet. 8, 123–128 - PubMed
-
- Tufan A. C., Satiroglu-Tufan N. L., Jackson G. C., Semerci C. N., Solak S., and Yagci B. (2007) Serum or plasma cartilage oligomeric matrix protein concentration as a diagnostic marker in pseudoachondroplasia: differential diagnosis of a family. Eur. J. Hum. Genet. 15, 1023–1028 - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

