IRAK4 kinase activity controls Toll-like receptor-induced inflammation through the transcription factor IRF5 in primary human monocytes
- PMID: 28924041
- PMCID: PMC5682975
- DOI: 10.1074/jbc.M117.796912
IRAK4 kinase activity controls Toll-like receptor-induced inflammation through the transcription factor IRF5 in primary human monocytes
Abstract
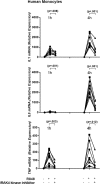
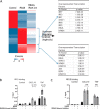
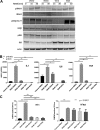
Interleukin-1 receptor-associated kinase 4 (IRAK4) plays a critical role in innate immune signaling by Toll-like receptors (TLRs), and loss of IRAK4 activity in mice and humans increases susceptibility to bacterial infections and causes defects in TLR and IL1 ligand sensing. However, the mechanism by which IRAK4 activity regulates the production of downstream inflammatory cytokines is unclear. Using transcriptomic and biochemical analyses of human monocytes treated with a highly potent and selective inhibitor of IRAK4, we show that IRAK4 kinase activity controls the activation of interferon regulatory factor 5 (IRF5), a transcription factor implicated in the pathogenesis of multiple autoimmune diseases. Following TLR7/8 stimulation by its agonist R848, chemical inhibition of IRAK4 abolished IRF5 translocation to the nucleus and thus prevented IRF5 binding to and activation of the promoters of inflammatory cytokines in human monocytes. We also found that IKKβ, an upstream IRF5 activator, is phosphorylated in response to the agonist-induced TLR signaling. Of note, IRAK4 inhibition blocked IKKβ phosphorylation but did not block the nuclear translocation of NFκB, which was surprising, given the canonical role of IKKβ in phosphorylating IκB to allow NFκB activation. Moreover, pharmacological inhibition of either IKKβ or the serine/threonine protein kinase TAK1 in monocytes blocked TLR-induced cytokine production and IRF5 translocation to the nucleus, but not nuclear translocation of NFκB. Taken together, our data suggest a mechanism by which IRAK4 activity regulates TAK1 and IKKβ activation, leading to the nuclear translocation of IRF5 and induction of inflammatory cytokines in human monocytes.
Keywords: IKKβ; IRAK4; IRF5; NFκB; TAK1; TLR; Toll-like receptor; cytokine; inflammation; interferon regulatory factor.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
W. K. and R. H. were employees of Nodality, contracted by Pfizer to perform SCNP studies with the IRAK4 inhibitor. All other authors were Pfizer employees
Figures


References
-
- Picard C., Puel A., Bonnet M., Ku C. L., Bustamante J., Yang K., Soudais C., Dupuis S., Feinberg J., Fieschi C., Elbim C., Hitchcock R., Lammas D., Davies G., Al-Ghonaium A., et al. (2003) Pyogenic bacterial infections in humans with IRAK-4 deficiency. Science 299, 2076–2079 - PubMed
-
- Suzuki N., Suzuki S., Duncan G. S., Millar D. G., Wada T., Mirtsos C., Takada H., Wakeham A., Itie A., Li S., Penninger J. M., Wesche H., Ohashi P. S., Mak T. W., and Yeh W.-C. (2002) Severe impairment of interleukin-1 and Toll-like receptor signalling in mice lacking IRAK-4. Nature 416, 750–756 - PubMed
-
- Seganish W. M. (2016) Inhibitors of interleukin-1 receptor-associated kinase 4 (IRAK4): A patent review. (2012–2015). Expert Opin. Ther. Pat. 26, 917–932 - PubMed
-
- Flannery S., and Bowie A. G. (2010) The interleukin-1 receptor-associated kinases: Critical regulators of innate immune signalling. Biochem. Pharmacol. 80, 1981–1991 - PubMed
-
- Cushing L., Stochaj W., Siegel M., Czerwinski R., Dower K., Wright Q., Hirschfield M., Casanova J. L., Picard C., Puel A., Lin L. L., and Rao V. R. (2014) Interleukin 1/Toll-like receptor-induced autophosphorylation activates interleukin 1 receptor-associated kinase 4 and controls cytokine induction in a cell type-specific manner. J. Biol. Chem. 289, 10865–10875 - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

