Alternative cleavage of the bone morphogenetic protein (BMP), Gbb, produces ligands with distinct developmental functions and receptor preferences
- PMID: 28924042
- PMCID: PMC5702660
- DOI: 10.1074/jbc.M117.793513
Alternative cleavage of the bone morphogenetic protein (BMP), Gbb, produces ligands with distinct developmental functions and receptor preferences
Abstract
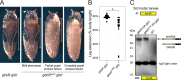
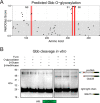
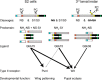
The family of TGF-β and bone morphogenetic protein (BMP) signaling proteins has numerous developmental and physiological roles. They are made as proprotein dimers and then cleaved by proprotein convertases to release the C-terminal domain as an active ligand dimer. Multiple proteolytic processing sites in Glass bottom boat (Gbb), the Drosophila BMP7 ortholog, can produce distinct ligand forms. Cleavage at the S1 or atypical S0 site in Gbb produces Gbb15, the conventional small BMP ligand, whereas NS site cleavage produces a larger Gbb38 ligand. We hypothesized that the Gbb prodomain is involved not only in regulating the production of specific ligands but also their signaling output. We found that blocking NS cleavage increased association of the full-length prodomain with Gbb15, resulting in a concomitant decrease in signaling activity. Moreover, NS cleavage was required in vivo for Gbb-Decapentaplegic (Dpp) heterodimer-mediated wing vein patterning but not for Gbb15-Dpp heterodimer activity in cell culture. Gbb NS cleavage was also required for viability through its regulation of pupal ecdysis in a type II receptor Wishful thinking (Wit)-dependent manner. In fact, Gbb38-mediated signaling exhibits a preference for Wit over the other type II receptor Punt. Finally, we discovered that Gbb38 is produced when processing at the S1/S0 site is blocked by O-linked glycosylation in third instar larvae. Our findings demonstrate that BMP prodomain cleavage ensures that the mature ligand is not inhibited by the prodomain. Furthermore, alternative processing of BMP proproteins produces ligands that signal through different receptors and exhibit specific developmental functions.
Keywords: Drosophila; O-GlcNAcylation; Glass bottom boat (Gbb); bone morphogenetic protein (BMP); cell signaling; prodomain; proprotein convertase; protein processing; transforming growth factor β (TGF-β).
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures







References
-
- Bragdon B., Moseychuk O., Saldanha S., King D., Julian J., and Nohe A. (2011) Bone morphogenetic proteins: a critical review. Cell. Signal. 23, 609–620 - PubMed
-
- Wharton K., and Derynck R. (2009) TGFβ family signaling: novel insights in development and disease. Development 136, 3691–3697 - PubMed
-
- Constam D. B. (2014) Regulation of TGFβ and related signals by precursor processing. Semin. Cell Dev. Biol. 32, 85–97 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

