Ephrin-A2 regulates excitatory neuron differentiation and interneuron migration in the developing neocortex
- PMID: 28924206
- PMCID: PMC5603509
- DOI: 10.1038/s41598-017-12185-x
Ephrin-A2 regulates excitatory neuron differentiation and interneuron migration in the developing neocortex
Abstract
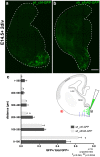
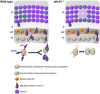
The development of the neocortex requires co-ordination between proliferation and differentiation, as well as the precise orchestration of neuronal migration. Eph/ephrin signaling is crucial in guiding neurons and their projections during embryonic development. In adult ephrin-A2 knockout mice we consistently observed focal patches of disorganized neocortical laminar architecture, ranging in severity from reduced neuronal density to a complete lack of neurons. Loss of ephrin-A2 in the pre-optic area of the diencephalon reduced the migration of neocortex-bound interneurons from this region. Furthermore, ephrin-A2 participates in the creation of excitatory neurons by inhibiting apical progenitor proliferation in the ventricular zone, with the disruption of ephrin-A2 signaling in these cells recapitulating the abnormal neocortex observed in the knockout. The disturbance to the architecture of the neocortex observed following deletion of ephrin-A2 signaling shares many similarities with defects found in the neocortex of children diagnosed with autism spectrum disorder.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
EphA/ephrin A reverse signaling promotes the migration of cortical interneurons from the medial ganglionic eminence.Development. 2014 Jan;141(2):460-71. doi: 10.1242/dev.101691. Development. 2014. PMID: 24381199
-
Autonomous and non-autonomous roles for ephrin-B in interneuron migration.Dev Biol. 2017 Nov 15;431(2):179-193. doi: 10.1016/j.ydbio.2017.09.024. Epub 2017 Sep 22. Dev Biol. 2017. PMID: 28947178 Free PMC article.
-
Ephrin-B2 paces neuronal production in the developing neocortex.BMC Dev Biol. 2020 May 13;20(1):12. doi: 10.1186/s12861-020-00215-3. BMC Dev Biol. 2020. PMID: 32404061 Free PMC article.
-
Transcriptional co-regulation of neuronal migration and laminar identity in the neocortex.Development. 2012 May;139(9):1535-46. doi: 10.1242/dev.069963. Development. 2012. PMID: 22492350 Free PMC article. Review.
-
Migratory pathways of GABAergic interneurons when they enter the neocortex.Eur J Neurosci. 2012 Jun;35(11):1655-60. doi: 10.1111/j.1460-9568.2012.08111.x. Epub 2012 May 28. Eur J Neurosci. 2012. PMID: 22639844 Review.
Cited by
-
Identification of genes associated with cortical malformation using a transposon-mediated somatic mutagenesis screen in mice.Nat Commun. 2018 Jun 27;9(1):2498. doi: 10.1038/s41467-018-04880-8. Nat Commun. 2018. PMID: 29950674 Free PMC article.
-
Master regulators of neurogenesis: the dynamic roles of Ephrin receptors across diverse cellular niches.Transl Psychiatry. 2024 Nov 6;14(1):462. doi: 10.1038/s41398-024-03168-4. Transl Psychiatry. 2024. PMID: 39505843 Free PMC article. Review.
-
Identification of age-specific gene regulators of La Crosse virus neuroinvasion and pathogenesis.Nat Commun. 2023 May 18;14(1):2836. doi: 10.1038/s41467-023-37833-x. Nat Commun. 2023. PMID: 37202395 Free PMC article.
-
Aryl hydrocarbon receptor is a tumor promoter in MYCN-amplified neuroblastoma cells through suppression of differentiation.iScience. 2023 Oct 21;26(11):108303. doi: 10.1016/j.isci.2023.108303. eCollection 2023 Nov 17. iScience. 2023. PMID: 38026169 Free PMC article.
-
Molecular Characterisation of the Mechanism of Action of Stimulant Drugs Lisdexamfetamine and Methylphenidate on ADHD Neurobiology: A Review.Neurol Ther. 2022 Dec;11(4):1489-1517. doi: 10.1007/s40120-022-00392-2. Epub 2022 Aug 11. Neurol Ther. 2022. PMID: 35951288 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

