Novel ecto-tagged integrins reveal their trafficking in live cells
- PMID: 28924207
- PMCID: PMC5603536
- DOI: 10.1038/s41467-017-00646-w
Novel ecto-tagged integrins reveal their trafficking in live cells
Abstract
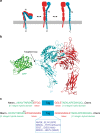
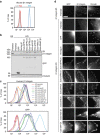
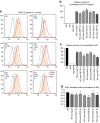
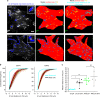
Integrins are abundant heterodimeric cell-surface adhesion receptors essential in multicellular organisms. Integrin function is dynamically modulated by endo-exocytic trafficking, however, major mysteries remain about where, when, and how this occurs in living cells. To address this, here we report the generation of functional recombinant β1 integrins with traceable tags inserted in an extracellular loop. We demonstrate that these 'ecto-tagged' integrins are cell-surface expressed, localize to adhesions, exhibit normal integrin activation, and restore adhesion in β1 integrin knockout fibroblasts. Importantly, β1 integrins containing an extracellular pH-sensitive pHluorin tag allow direct visualization of integrin exocytosis in live cells and revealed targeted delivery of integrin vesicles to focal adhesions. Further, using β1 integrins containing a HaloTag in combination with membrane-permeant and -impermeant Halo dyes allows imaging of integrin endocytosis and recycling. Thus, ecto-tagged integrins provide novel powerful tools to characterize integrin function and trafficking.Integrins are cell-surface adhesion receptors that are modulated by endo-exocytic trafficking, but existing tools to study this process can interfere with function. Here the authors develop β1 integrins carrying traceable tags in the extracellular domain; a pH-sensitive pHlourin tag or a HaloTag to facilitate dye attachment.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



Similar articles
-
Use of Ecto-Tagged Integrins to Monitor Integrin Exocytosis and Endocytosis.Methods Mol Biol. 2023;2608:17-38. doi: 10.1007/978-1-0716-2887-4_2. Methods Mol Biol. 2023. PMID: 36653699 Free PMC article.
-
Liprin-alpha1 affects the distribution of low-affinity beta1 integrins and stabilizes their permanence at the cell surface.Exp Cell Res. 2010 Apr 1;316(6):915-26. doi: 10.1016/j.yexcr.2010.01.017. Epub 2010 Jan 22. Exp Cell Res. 2010. PMID: 20096687
-
Calcium and calmodulin-dependent serine/threonine protein kinase type II (CaMKII)-mediated intramolecular opening of integrin cytoplasmic domain-associated protein-1 (ICAP-1α) negatively regulates β1 integrins.J Biol Chem. 2013 Jul 12;288(28):20248-60. doi: 10.1074/jbc.M113.455956. Epub 2013 May 28. J Biol Chem. 2013. PMID: 23720740 Free PMC article.
-
Mechanisms of integrin activation and trafficking.Curr Opin Cell Biol. 2011 Oct;23(5):607-14. doi: 10.1016/j.ceb.2011.08.005. Epub 2011 Sep 14. Curr Opin Cell Biol. 2011. PMID: 21924601 Review.
-
On the move: endocytic trafficking in cell migration.Cell Mol Life Sci. 2015 Jun;72(11):2119-34. doi: 10.1007/s00018-015-1855-9. Epub 2015 Feb 15. Cell Mol Life Sci. 2015. PMID: 25681867 Free PMC article. Review.
Cited by
-
Listeria monocytogenes exploits host exocytosis to promote cell-to-cell spread.Proc Natl Acad Sci U S A. 2020 Feb 18;117(7):3789-3796. doi: 10.1073/pnas.1916676117. Epub 2020 Feb 3. Proc Natl Acad Sci U S A. 2020. PMID: 32015134 Free PMC article.
-
Role of Site-Specific Glycosylation in the I-Like Domain of Integrin β1 in Small Extracellular Vesicle-Mediated Malignant Behavior and FAK Activation.Int J Mol Sci. 2021 Feb 10;22(4):1770. doi: 10.3390/ijms22041770. Int J Mol Sci. 2021. PMID: 33578954 Free PMC article.
-
Integrin trafficking in cells and tissues.Nat Cell Biol. 2019 Feb;21(2):122-132. doi: 10.1038/s41556-018-0223-z. Epub 2019 Jan 2. Nat Cell Biol. 2019. PMID: 30602723 Free PMC article. Review.
-
Cooperation of cell adhesion and autophagy in the brain: Functional roles in development and neurodegenerative disease.Matrix Biol Plus. 2021 Oct 23;12:100089. doi: 10.1016/j.mbplus.2021.100089. eCollection 2021 Dec. Matrix Biol Plus. 2021. PMID: 34786551 Free PMC article. Review.
-
Tumor-Derived Extracellular Vesicles Require β1 Integrins to Promote Anchorage-Independent Growth.iScience. 2019 Apr 26;14:199-209. doi: 10.1016/j.isci.2019.03.022. Epub 2019 Mar 27. iScience. 2019. PMID: 30981115 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

