Organocatalytic synthesis of chiral tetrasubstituted allenes from racemic propargylic alcohols
- PMID: 28924216
- PMCID: PMC5603569
- DOI: 10.1038/s41467-017-00251-x
Organocatalytic synthesis of chiral tetrasubstituted allenes from racemic propargylic alcohols
Abstract
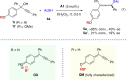
Although chiral allene preparation via formal SN2' nucleophilic substitutions of enantioenriched propargylic derivatives or metal-catalyzed reactions of racemic propargylic derivatives has attracted considerable attention and found applications in many areas of research, direct use of propargylic alcohols instead of propargylic derivatives for catalytic asymmetric allene synthesis is unknown. Here, we show that a highly enantioselective synthesis of tetrasubstituted allenes from racemic propargylic alcohols has been realized by organocatalysis with good efficiency (up to 96% yield and 97% ee). The intermolecular C-C and C-S bond formation was achieved efficiently with simultaneous stereocontrol over the axial chirality. Furthermore, an adjacent quaternary stereocenter could also be constructed. Mechanistically, the reaction may involve efficient stereocontrol on the propargylic cation by its chiral counter anion or 1,8-conjugate addition of para-quinone methides. In sharp contrast to previous central chirality construction, this process employs quinone methides for axial chirality construction.Axially chiral allenes that are normally present in natural products, bioactive molecules, organocatalysts, and functional materials are usually produced from propargylic derivatives. Here, the authors show direct use of propargylic alcohols for catalytic asymmetric allene synthesis.
Conflict of interest statement
The authors declare no competing financial interests.
Figures





Similar articles
-
Organocatalytic synthesis of axially chiral tetrasubstituted allenes.Org Biomol Chem. 2023 Jan 4;21(2):252-272. doi: 10.1039/d2ob01794f. Org Biomol Chem. 2023. PMID: 36504200 Review.
-
Organocatalytic Enantioselective Synthesis of Chiral Allenes: Remote Asymmetric 1,8-Addition of Indole Imine Methides.Angew Chem Int Ed Engl. 2020 Sep 21;59(39):17049-17054. doi: 10.1002/anie.202006137. Epub 2020 Jul 28. Angew Chem Int Ed Engl. 2020. PMID: 32558012
-
Construction of Axially Chiral Compounds via Asymmetric Organocatalysis.Acc Chem Res. 2018 Feb 20;51(2):534-547. doi: 10.1021/acs.accounts.7b00602. Epub 2018 Feb 8. Acc Chem Res. 2018. PMID: 29419282
-
Organocatalytic Remote Stereocontrolled 1,8-Additions of Thiazolones to Propargylic Aza-p-quinone Methides.Org Lett. 2019 Sep 20;21(18):7415-7419. doi: 10.1021/acs.orglett.9b02726. Epub 2019 Sep 5. Org Lett. 2019. PMID: 31486650
-
Construction of Axially Chiral Compounds via Central-to-Axial Chirality Conversion.Chem Asian J. 2020 Oct 1;15(19):2939-2951. doi: 10.1002/asia.202000681. Epub 2020 Aug 28. Chem Asian J. 2020. PMID: 32755013 Review.
Cited by
-
Primary activation of para-quinone methides by chiral phosphoric acid for enantioselective construction of tetraarylmethanes.Chem Sci. 2023 Dec 2;15(2):720-725. doi: 10.1039/d3sc05014a. eCollection 2024 Jan 3. Chem Sci. 2023. PMID: 38179542 Free PMC article.
-
Enantiodivergent Synthesis of Allenes by Point-to-Axial Chirality Transfer.Angew Chem Int Ed Engl. 2018 Jul 2;57(27):8203-8208. doi: 10.1002/anie.201804446. Epub 2018 May 30. Angew Chem Int Ed Engl. 2018. PMID: 29719111 Free PMC article.
-
Brønsted acid-catalyzed asymmetric dearomatization for synthesis of chiral fused polycyclic enone and indoline scaffolds.Sci Adv. 2023 Mar 17;9(11):eadg4648. doi: 10.1126/sciadv.adg4648. Epub 2023 Mar 15. Sci Adv. 2023. PMID: 36921050 Free PMC article.
-
Mechanistic Aspects and Synthetic Applications of Radical Additions to Allenes.Chem Rev. 2019 Dec 26;119(24):12422-12490. doi: 10.1021/acs.chemrev.9b00312. Epub 2019 Dec 13. Chem Rev. 2019. PMID: 31833759 Free PMC article.
-
Copper(I)-catalyzed diastereo- and enantio-selective construction of optically pure exocyclic allenes.Nat Commun. 2020 Aug 27;11(1):4293. doi: 10.1038/s41467-020-18136-x. Nat Commun. 2020. PMID: 32855405 Free PMC article.
References
-
- Krause, N. & Hashmi A. S. K. Modern Allene Chemistry (Wiley, 2004).
-
- Ogasawara M. Catalytic enantioselective synthesis of axially chiral allenes. Tetrahedron: asymmetry. 2009;20:259–271. doi: 10.1016/j.tetasy.2008.11.039. - DOI
-
- Ye J-T, Ma S. Conquering three-carbon axial chirality of allenes. Org. Chem. Front. 2014;1:1210–1224. doi: 10.1039/C4QO00208C. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

