Residual Ammonium Persulfate in Nanoparticles Has Cytotoxic Effects on Cells through Epithelial-Mesenchymal Transition
- PMID: 28924225
- PMCID: PMC5603593
- DOI: 10.1038/s41598-017-12328-0
Residual Ammonium Persulfate in Nanoparticles Has Cytotoxic Effects on Cells through Epithelial-Mesenchymal Transition
Abstract
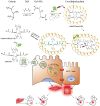
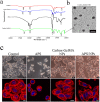
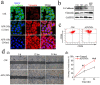
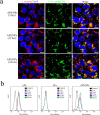
Ammonium persulfate (APS), a low molecular weight chemical compound with strong oxidizing properties, should to be totally removed during preparation of nanomaterials due to its cytotoxicity. APS exerts its oxidative stress effects mainly on cell membrane, but its intracellular influence remains unclear. Here, we designed a facile negatively-charged carboxylic gelatin-methyacrylate (carbox-GelMA) nanoparticle (NP) as a cargo-carrier through the catalytic and oxidizing action of APS in W/O system. The formed APS-loaded carbox-GelMA NPs (APS/NPs) were transported into the lysosome in MCF-7 breast cancer cells. The intracellular APS/NPs produced a high level of oxidative stress in lysosome and induced epithelial-mesenchymal transition (EMT). Consequently, the MCF-7 cells challenged with APS/NPs had a strong metastatic and invasive capability in vitro and in vivo. This study highlights that a facile APS-loaded nanocarrier has cyctotoxicity on cells through EMT. Unexpectedly, we found a novel pathway inducing EMT via lysosomal oxidative stress.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Ammonium Persulfate-Loaded Carboxylic Gelatin-Methacrylate Nanoparticles Promote Cardiac Repair by Activating Epicardial Epithelial-Mesenchymal Transition via Autophagy and the mTOR Pathway.ACS Nano. 2023 Oct 24;17(20):20246-20261. doi: 10.1021/acsnano.3c06229. Epub 2023 Oct 2. ACS Nano. 2023. PMID: 37782701
-
Fludioxonil induced the cancer growth and metastasis via altering epithelial-mesenchymal transition via an estrogen receptor-dependent pathway in cellular and xenografted breast cancer models.Environ Toxicol. 2017 Apr;32(4):1439-1454. doi: 10.1002/tox.22337. Epub 2016 Aug 19. Environ Toxicol. 2017. PMID: 27539251
-
Generation of MCF-7 cells with aggressive metastatic potential in vitro and in vivo.Breast Cancer Res Treat. 2014 Nov;148(2):269-77. doi: 10.1007/s10549-014-3159-4. Epub 2014 Oct 8. Breast Cancer Res Treat. 2014. PMID: 25292421
-
Epithelial to mesenchymal transition is associated with rapamycin resistance.Oncotarget. 2015 Aug 14;6(23):19500-13. doi: 10.18632/oncotarget.3669. Oncotarget. 2015. PMID: 25944619 Free PMC article.
-
Fucoidan induces changes in the epithelial to mesenchymal transition and decreases metastasis by enhancing ubiquitin-dependent TGFβ receptor degradation in breast cancer.Carcinogenesis. 2013 Apr;34(4):874-84. doi: 10.1093/carcin/bgs396. Epub 2012 Dec 28. Carcinogenesis. 2013. PMID: 23275155
Cited by
-
In vitro cytotoxicity of resin cement and its influence on the expression of antioxidant genes.Acta Odontol Latinoam. 2023 Aug 31;36(2):120-127. doi: 10.54589/aol.36/2/. Acta Odontol Latinoam. 2023. PMID: 37776509 Free PMC article.
-
The Lifespan of D. melanogaster Depends on the Function of the Gagr Gene, a Domesticated gag Gene of Drosophila LTR Retrotransposons.Insects. 2024 Jan 17;15(1):68. doi: 10.3390/insects15010068. Insects. 2024. PMID: 38249074 Free PMC article.
-
Non-cytotoxic Dityrosine Photocrosslinked Polymeric Materials With Targeted Elastic Moduli.Front Chem. 2020 Mar 13;8:173. doi: 10.3389/fchem.2020.00173. eCollection 2020. Front Chem. 2020. PMID: 32232027 Free PMC article.
-
GelMA Core-Shell Microgel Preparation Based on a Droplet Microfluidic Device for Three-Dimensional Tumor Ball Culture and Its Drug Testing.Molecules. 2025 Aug 7;30(15):3305. doi: 10.3390/molecules30153305. Molecules. 2025. PMID: 40807480 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

