The autophagy initiator ULK1 sensitizes AMPK to allosteric drugs
- PMID: 28924239
- PMCID: PMC5603566
- DOI: 10.1038/s41467-017-00628-y
The autophagy initiator ULK1 sensitizes AMPK to allosteric drugs
Abstract
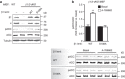
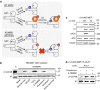
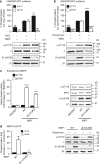
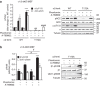
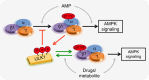
AMP-activated protein kinase (AMPK) is a metabolic stress-sensing enzyme responsible for maintaining cellular energy homeostasis. Activation of AMPK by salicylate and the thienopyridone A-769662 is critically dependent on phosphorylation of Ser108 in the β1 regulatory subunit. Here, we show a possible role for Ser108 phosphorylation in cell cycle regulation and promotion of pro-survival pathways in response to energy stress. We identify the autophagy initiator Unc-51-like kinase 1 (ULK1) as a β1-Ser108 kinase in cells. Cellular β1-Ser108 phosphorylation by ULK1 was dependent on AMPK β-subunit myristoylation, metabolic stress associated with elevated AMP/ATP ratio, and the intrinsic energy sensing capacity of AMPK; features consistent with an AMP-induced myristoyl switch mechanism. We further demonstrate cellular AMPK signaling independent of activation loop Thr172 phosphorylation, providing potential insight into physiological roles for Ser108 phosphorylation. These findings uncover new mechanisms by which AMPK could potentially maintain cellular energy homeostasis independently of Thr172 phosphorylation.AMPK is involved in sensing of metabolic stress. The authors show that the autophagy initiator ULK1 phosphorylates β1-Ser108 on the regulatory β1-subunit, sensitizing AMPK to allosteric drugs, and activates signaling pathways that appear independent of Thr172 phosphorylation in the kinase activation loop.
Conflict of interest statement
The authors declare no competing financial interests.
Figures




Similar articles
-
Small molecule drug A-769662 and AMP synergistically activate naive AMPK independent of upstream kinase signaling.Chem Biol. 2014 May 22;21(5):619-27. doi: 10.1016/j.chembiol.2014.03.006. Epub 2014 Apr 17. Chem Biol. 2014. PMID: 24746562
-
β-Subunit myristoylation is the gatekeeper for initiating metabolic stress sensing by AMP-activated protein kinase (AMPK).Proc Natl Acad Sci U S A. 2010 Nov 9;107(45):19237-41. doi: 10.1073/pnas.1009705107. Epub 2010 Oct 25. Proc Natl Acad Sci U S A. 2010. PMID: 20974912 Free PMC article.
-
ULK1-regulated AMP sensing by AMPK and its application for the treatment of chronic kidney disease.Kidney Int. 2024 Nov;106(5):887-906. doi: 10.1016/j.kint.2024.08.024. Kidney Int. 2024. PMID: 39428173
-
Role of an intrinsically disordered conformation in AMPK-mediated phosphorylation of ULK1 and regulation of autophagy.Mol Biosyst. 2012 Jan;8(1):91-6. doi: 10.1039/c1mb05265a. Epub 2011 Aug 18. Mol Biosyst. 2012. PMID: 21853163 Review.
-
Structural and biochemical insights into the allosteric activation mechanism of AMP-activated protein kinase.Chem Biol Drug Des. 2017 May;89(5):663-669. doi: 10.1111/cbdd.12897. Epub 2016 Dec 9. Chem Biol Drug Des. 2017. PMID: 27809416 Review.
Cited by
-
ALDOA maintains NLRP3 inflammasome activation by controlling AMPK activation.Autophagy. 2022 Jul;18(7):1673-1693. doi: 10.1080/15548627.2021.1997051. Epub 2021 Nov 25. Autophagy. 2022. PMID: 34821530 Free PMC article.
-
New developments in AMPK and mTORC1 cross-talk.Essays Biochem. 2024 Nov 18;68(3):321-336. doi: 10.1042/EBC20240007. Essays Biochem. 2024. PMID: 38994736 Free PMC article. Review.
-
Metformin attenuates cartilage degeneration in an experimental osteoarthritis model by regulating AMPK/mTOR.Aging (Albany NY). 2020 Jan 16;12(2):1087-1103. doi: 10.18632/aging.102635. Epub 2020 Jan 16. Aging (Albany NY). 2020. PMID: 31945013 Free PMC article.
-
AMP-activated protein kinase selectively inhibited by the type II inhibitor SBI-0206965.J Biol Chem. 2018 Jun 8;293(23):8874-8885. doi: 10.1074/jbc.RA118.003547. Epub 2018 Apr 25. J Biol Chem. 2018. PMID: 29695504 Free PMC article.
-
Triclosan induces ROS-dependent cell death and autophagy in A375 melanoma cells.Oncol Lett. 2020 Oct;20(4):73. doi: 10.3892/ol.2020.11934. Epub 2020 Jul 30. Oncol Lett. 2020. PMID: 32863906 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

