Refinement of ectopic protein expression through the GAL4/UAS system in Bombyx mori: application to behavioral and developmental studies
- PMID: 28924263
- PMCID: PMC5603595
- DOI: 10.1038/s41598-017-12102-2
Refinement of ectopic protein expression through the GAL4/UAS system in Bombyx mori: application to behavioral and developmental studies
Abstract
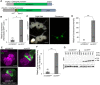
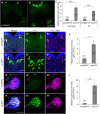
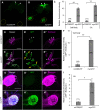
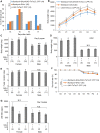
Silkmoth, Bombyx mori, is one of the important model insects in which transgenic techniques and the GAL4/UAS system are applicable. However, due to cytotoxicity and low transactivation activity of GAL4, effectiveness of the GAL4/UAS system and its application in B. mori are still limited. In the present study, we refined the previously reported UAS vector by exploiting transcriptional and translational enhancers, and achieved 200-fold enhancement of reporter GFP fluorescence in the GAL4/UAS system. Enhanced protein expression of membrane-targeted GFP and calcium indicator protein (GCaMP5G) drastically improved visualization of fine neurite structures and neural activity, respectively. Also, with the refined system, we generated a transgenic strain that expresses tetanus toxin light chain (TeTxLC), which blocks synaptic transmission, under the control of GAL4. Ectopic TeTxLC expression in the sex pheromone receptor neurons inhibited male courtship behavior, proving effectiveness of TeTxLC on loss-of-function analyses of neural circuits. In addition, suppression of prothoracicotropic hormone (PTTH) or insulin-like peptide (bombyxin) secretion impaired developmental timing and growth rate, respectively. Furthermore, we revealed that larval growth is sex-differentially regulated by these peptide hormones. The present study provides important technical underpinnings of transgenic approaches in silkmoths and insights into mechanisms of postembryonic development in insects.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




Similar articles
-
Insights into the regulatory characteristics of silkworm fibroin gene promoters using a modified Gal4/UAS system.Transgenic Res. 2019 Dec;28(5-6):627-636. doi: 10.1007/s11248-019-00175-w. Epub 2019 Sep 29. Transgenic Res. 2019. PMID: 31564030
-
Establishment of tools for neurogenetic analysis of sexual behavior in the silkmoth, Bombyx mori.PLoS One. 2014 Nov 14;9(11):e113156. doi: 10.1371/journal.pone.0113156. eCollection 2014. PLoS One. 2014. PMID: 25396742 Free PMC article.
-
GFP labeling of neurosecretory cells with the GAL4/UAS system in the silkmoth brain enables selective intracellular staining of neurons.Zoolog Sci. 2008 May;25(5):509-16. doi: 10.2108/zsj.25.509. Zoolog Sci. 2008. PMID: 18558804
-
The brain secretory peptides that control moulting and metamorphosis of the silkmoth, Bombyx mori.Int J Dev Biol. 1994 Jun;38(2):301-10. Int J Dev Biol. 1994. PMID: 7981038 Review.
-
Gal4 Driver Transgenic Zebrafish: Powerful Tools to Study Developmental Biology, Organogenesis, and Neuroscience.Adv Genet. 2016;95:65-87. doi: 10.1016/bs.adgen.2016.04.002. Epub 2016 Jun 13. Adv Genet. 2016. PMID: 27503354 Review.
Cited by
-
An overview of functional genomic tools in deciphering insecticide resistance.Curr Opin Insect Sci. 2018 Jun;27:103-110. doi: 10.1016/j.cois.2018.04.004. Epub 2018 Apr 13. Curr Opin Insect Sci. 2018. PMID: 30025625 Free PMC article. Review.
-
Insights into the regulatory characteristics of silkworm fibroin gene promoters using a modified Gal4/UAS system.Transgenic Res. 2019 Dec;28(5-6):627-636. doi: 10.1007/s11248-019-00175-w. Epub 2019 Sep 29. Transgenic Res. 2019. PMID: 31564030
-
Activity-Dependent Remodeling of Drosophila Olfactory Sensory Neuron Brain Innervation during an Early-Life Critical Period.J Neurosci. 2019 Apr 17;39(16):2995-3012. doi: 10.1523/JNEUROSCI.2223-18.2019. Epub 2019 Feb 12. J Neurosci. 2019. PMID: 30755492 Free PMC article.
-
An insulin-like growth factor-like peptide promotes ovarian development in the silkmoth Bombyx mori.Sci Rep. 2019 Dec 5;9(1):18446. doi: 10.1038/s41598-019-54962-w. Sci Rep. 2019. PMID: 31804598 Free PMC article.
-
Genetic and viral approaches to record or manipulate neurons in insects.Curr Opin Insect Sci. 2021 Dec;48:79-88. doi: 10.1016/j.cois.2021.10.003. Epub 2021 Oct 25. Curr Opin Insect Sci. 2021. PMID: 34710643 Free PMC article. Review.
References
-
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development (Cambridge, England) 1993;118:401–415. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

