Linker Histone in Diseases
- PMID: 28924382
- PMCID: PMC5599906
- DOI: 10.7150/ijbs.19891
Linker Histone in Diseases
Abstract
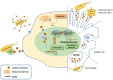
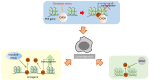
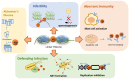
The linker histone is a protein that binds with the nucleosome, which is generally considered to achieve chromatin condensation in the nucleus. Accumulating evidences suggest that the linker histone is essential in the pathogenesis of several diseases. In this review, we briefly introduce the current knowledge of the linker histone, including its structure, characteristics and functions. Also, we move forward to present the advances of the linker histone's association with certain diseases, such as cancer, Alzheimer's disease, infection, male infertility and aberrant immunity situations, focusing on the alteration of the linker histone under certain pathological conditions and its role in developing each disease.
Keywords: Alzheimer's Disease.; Cancer; Chromatin; Linker Histone; Nucleosome.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures
Similar articles
-
A quantitative investigation of linker histone interactions with nucleosomes and chromatin.Sci Rep. 2016 Jan 11;6:19122. doi: 10.1038/srep19122. Sci Rep. 2016. PMID: 26750377 Free PMC article.
-
Complex of linker histone H5 with the nucleosome and its implications for chromatin packing.Proc Natl Acad Sci U S A. 2006 May 30;103(22):8384-9. doi: 10.1073/pnas.0508951103. Epub 2006 May 22. Proc Natl Acad Sci U S A. 2006. PMID: 16717183 Free PMC article.
-
HMGN1 and 2 remodel core and linker histone tail domains within chromatin.Nucleic Acids Res. 2017 Sep 29;45(17):9917-9930. doi: 10.1093/nar/gkx579. Nucleic Acids Res. 2017. PMID: 28973435 Free PMC article.
-
Linker histones: novel insights into structure-specific recognition of the nucleosome.Biochem Cell Biol. 2017 Apr;95(2):171-178. doi: 10.1139/bcb-2016-0097. Epub 2016 Jun 29. Biochem Cell Biol. 2017. PMID: 28177778 Free PMC article. Review.
-
Dynamic fuzziness during linker histone action.Adv Exp Med Biol. 2012;725:15-26. doi: 10.1007/978-1-4614-0659-4_2. Adv Exp Med Biol. 2012. PMID: 22399316 Review.
Cited by
-
Linker histone H1 modulates defense priming and immunity in plants.Nucleic Acids Res. 2023 May 22;51(9):4252-4265. doi: 10.1093/nar/gkad106. Nucleic Acids Res. 2023. PMID: 36840717 Free PMC article.
-
A Human Pan-Cancer System Analysis of Procollagen-Lysine, 2-Oxoglutarate 5-Dioxygenase 3 (PLOD3).Int J Mol Sci. 2021 Sep 14;22(18):9903. doi: 10.3390/ijms22189903. Int J Mol Sci. 2021. PMID: 34576068 Free PMC article.
-
Previously Unidentified Histone H1-Like Protein Is Involved in Cell Division and Ribosome Biosynthesis in Toxoplasma gondii.mSphere. 2022 Dec 21;7(6):e0040322. doi: 10.1128/msphere.00403-22. Epub 2022 Dec 5. mSphere. 2022. PMID: 36468865 Free PMC article.
-
Post-Translation Modifications and Mutations of Human Linker Histone Subtypes: Their Manifestation in Disease.Int J Mol Sci. 2023 Jan 11;24(2):1463. doi: 10.3390/ijms24021463. Int J Mol Sci. 2023. PMID: 36674981 Free PMC article. Review.
-
Interactome of intact chromatosome variants with site-specifically ubiquitylated and acetylated linker histone H1.2.Nucleic Acids Res. 2024 Jan 11;52(1):101-113. doi: 10.1093/nar/gkad1113. Nucleic Acids Res. 2024. PMID: 37994785 Free PMC article.
References
-
- Watson JD, Gann A, Baker TA, Bell SP, Levine M, Losick R. Molecular Biology of the Gene. Cold Spring Harbor Laboratory Press; 1987.
-
- Crane-Robinson C. Linker histones: History and current perspectives. Biochim Biophys Acta. 2016;1859:431–5. - PubMed
-
- Roque A, Ponte I, Suau P. Interplay between histone H1 structure and function. Biochim Biophys Acta. 2016;1859:444–54. - PubMed
-
- Cerf C, Lippens G, Muyldermans S, Segers A, Ramakrishnan V, Wodak SJ. et al. Homo- and heteronuclear two-dimensional NMR studies of the globular domain of histone H1: sequential assignment and secondary structure. Biochemistry. 1993;32:11345–51. - PubMed
-
- Ramakrishnan V, Finch JT, Graziano V, Lee PL, Sweet RM. Crystal structure of globular domain of histone H5 and its implications for nucleosome binding. Nature. 1993;362:219–23. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

