FGFR3 deficient mice have accelerated fracture repair
- PMID: 28924384
- PMCID: PMC5599908
- DOI: 10.7150/ijbs.19309
FGFR3 deficient mice have accelerated fracture repair
Abstract
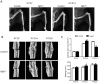
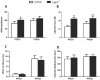
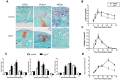
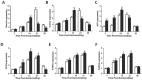
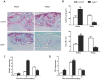
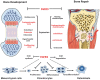
Bone fracture healing is processed through multiple biological stages that partly recapitulates the skeletal development process. FGFR3 is a negative regulator of chondrogenesis during embryonic stage and plays an important role in both chondrogenesis and osteogenesis. We have investigated the role of FGFR3 in fracture healing using unstabilized fracture model and found that gain-of-function mutation of FGFR3 inhibits the initiation of chondrogenesis during cartilage callus formation. Here, we created closed, stabilized proximal tibia fractures with an intramedullary pin in Fgfr3-/-mice and their littermate wild-type mice. Fracture healing was evaluated by radiography, micro-CT, histology, and real-time polymerase chain reaction (RT-PCR) analysis. The fractured Fgfr3-/- mice had increased formation of cartilaginous callus, more fracture callus, and more rapid endochondral ossification in fracture sites with up-regulated expressions of chondrogenesis related gene. The fractures of Fgfr3-/- mice healed faster with accelerated fracture callus mineralization and up-regulated expression of osteoblastogenic genes. The healing of fractures in Fgfr3-/- mice was accelerated in the stage of formation of cartilage and endochondral ossification. Downregulation of FGFR3 activity can be considered as a potential bio-therapeutic strategy for fracture treatment.
Keywords: FGFR3; fracture treatment.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures
Similar articles
-
PTH 1-34 Ameliorates the Osteopenia and Delayed Healing of Stabilized Tibia Fracture in Mice with Achondroplasia Resulting from Gain-Of-Function Mutation of FGFR3.Int J Biol Sci. 2017 Sep 21;13(10):1254-1265. doi: 10.7150/ijbs.21258. eCollection 2017. Int J Biol Sci. 2017. PMID: 29104492 Free PMC article.
-
Gain-of-function mutation of FGFR3 results in impaired fracture healing due to inhibition of chondrocyte differentiation.Biochem Biophys Res Commun. 2008 Nov 21;376(3):454-9. doi: 10.1016/j.bbrc.2008.08.165. Epub 2008 Sep 24. Biochem Biophys Res Commun. 2008. PMID: 18789890
-
Expression of fibroblast growth factor receptor-3 (FGFR3), signal transducer and activator of transcription-1, and cyclin-dependent kinase inhibitor p21 during endochondral ossification: differential role of FGFR3 in skeletal development and fracture repair.Endocrinology. 2003 Oct;144(10):4659-68. doi: 10.1210/en.2003-0158. Epub 2003 Jul 3. Endocrinology. 2003. PMID: 12960068
-
[Hereditary skeletal dysplasias and FGFR3 and PTHR1 signaling pathways].Med Sci (Paris). 2005 Nov;21(11):954-61. doi: 10.1051/medsci/20052111954. Med Sci (Paris). 2005. PMID: 16274647 Review. French.
-
Role of bone regeneration and turnover modulators in control of fracture.Crit Rev Eukaryot Gene Expr. 2007;17(3):197-213. doi: 10.1615/critreveukargeneexpr.v17.i3.30. Crit Rev Eukaryot Gene Expr. 2007. PMID: 17725489 Review.
Cited by
-
Infigratinib, a selective FGFR1-3 tyrosine kinase inhibitor, alters dentoalveolar development at high doses.Dev Dyn. 2023 Dec;252(12):1428-1448. doi: 10.1002/dvdy.642. Epub 2023 Jul 12. Dev Dyn. 2023. PMID: 37435833 Free PMC article.
-
A new method for segmentation and analysis of bone callus in rodent fracture models using micro-CT.J Orthop Res. 2023 Aug;41(8):1717-1728. doi: 10.1002/jor.25507. Epub 2023 Jan 11. J Orthop Res. 2023. PMID: 36582023 Free PMC article.
-
New developments in the biology of fibroblast growth factors.WIREs Mech Dis. 2022 Jul;14(4):e1549. doi: 10.1002/wsbm.1549. Epub 2022 Feb 9. WIREs Mech Dis. 2022. PMID: 35142107 Free PMC article. Review.
-
Comparative transcriptome analysis of the main beam and brow tine of sika deer antler provides insights into the molecular control of rapid antler growth.Cell Mol Biol Lett. 2020 Sep 7;25:42. doi: 10.1186/s11658-020-00234-9. eCollection 2020. Cell Mol Biol Lett. 2020. PMID: 32944020 Free PMC article.
-
Expansion of FGFR3-positive nucleus pulposus cells plays important roles in postnatal nucleus pulposus growth and regeneration.Stem Cell Res Ther. 2022 Jun 3;13(1):227. doi: 10.1186/s13287-022-02903-2. Stem Cell Res Ther. 2022. PMID: 35659742 Free PMC article.
References
-
- Shapiro F. Bone development and its relation to fracture repair. The role of mesenchymal osteoblasts and surface osteoblasts. Eur Cell Mater. 2008;15:53–76. - PubMed
-
- Vortkamp A, Pathi S, Peretti GM, Caruso EM, Zaleske DJ, Tabin CJ. Recapitulation of signals regulating embryonic bone formation during postnatal growth and in fracture repair. Mechanisms of development. 1998;71:65–76. - PubMed
-
- Chen Y, Alman BA. Wnt pathway, an essential role in bone regeneration. Journal of cellular biochemistry. 2009;106:353–62. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

