Age-Dependent Alpha-Synuclein Accumulation and Phosphorylation in the Enteric Nervous System in a Transgenic Mouse Model of Parkinson's Disease
- PMID: 28924920
- PMCID: PMC5636741
- DOI: 10.1007/s12264-017-0179-1
Age-Dependent Alpha-Synuclein Accumulation and Phosphorylation in the Enteric Nervous System in a Transgenic Mouse Model of Parkinson's Disease
Abstract
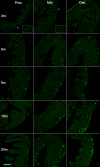
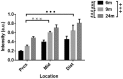
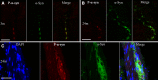
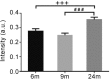
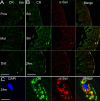
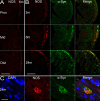
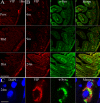
The enteric nervous system (ENS) controls the function of the gastrointestinal tract and has been implicated in various diseases, including Parkinson's disease (PD). PD is a neurodegenerative disease with Lewy bodies (LBs) and Lewy neurites (LNs) as the main pathological features. In addition to the typical motor symptoms in PD, attention has been drawn to non-motor symptoms, such as constipation, implying dysfunction of the ENS. In the present study, we characterized the age-dependent morphological alterations and aggregation of α-synuclein (α-syn), the primary protein component in LBs and LNs, in the ENS in an α-syn transgenic mouse model. We found that the expression and accumulation of α-syn increased gradually in neurons of Meissner's and Auerbach's plexuses of the gastrointestinal tract with age (from 1 week to 2 years). In addition, α-syn was increasingly phosphorylated at the serine 129 residue, reflecting pathological alterations of the protein over time. Furthermore, α-syn was present in different subtypes of neurons expressing vasoactive intestinal polypeptide, neuronal nitric oxide synthase, or calretinin. The results indicated that BAC-α-Syn-GFP transgenic mice provide a unique model in which to study the relationship between ENS and PD pathogenesis.
Keywords: Enteric system; Parkinson’s disease; Phosphorylation; Protein aggregation; α-synuclein.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

